The post Galectin Inhibitor Therapy Effective in Severe Atopic Dermatitis (Eczema) appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>Guest Author
I welcome as a co-author to this Perspective, Dr. Simon Ritchie, our lead investigator in skin disease and staff dermatologist, at San Antonio Military Health System, Fort Sam Houston, TX. Dr. Ritchie conducted the clinical study in moderate-to-severe plaque psoriasis and will present the promising results of the effect of our galectin inhibitor GR-MD-02 in psoriasis at the Maui Derm meeting March 20-23. The progression of results in the treatment of psoriasis has been previously released on March 6, 2016, May 16, 2016, and November 10, 2016.
What is atopic dermatitis (Eczema)
Atopic dermatitis, commonly called eczema, is a chronic inflammatory condition of the skin of caused by multiple factors that usually arises in early childhood, often in infancy. While it usually resolves by early teenage years, approximately 5-10% of patients have the disease extend into adulthood. As an allergic type of disorder, it is associated with asthma and seasonal allergies, which often occur in the same patient called the “atopic triad”. Atopic dermatitis is the itch that becomes a rash. Classic symptoms are itching and burning of the skin, resulting in thickening of the skin in response to the scratching. In some adults, it can be severe with debilitating itching, inability to sleep, and social stigmatization due to skin damage and thickening, often on the face.
How big a problem is atopic dermatitis?
Surveys suggest that up to 18% of the population have atopic dermatitis, up to 37% of those people seek medical care, and over 70% of those seeking care have mild disease that is handled by primary care physicians. Of those patients who seek medical care, approximately 20% have moderate and 2% have severe disease, which is generally referred to specialists. The national estimated cost of treatment is as high as $3.8 billion, as of 2012 (1).
No new therapies for difficult-to-treat atopic dermatitis in 30 years
Mild atopic dermatitis can be treated with skin moisturizers and topical steroid preparations, but there are fewer options for those that have more severe disease and do not respond. The only FDA approved systemic therapy is oral steroids, but multiple other drugs and approaches are often tried in clinical practice with limited efficacy and significant side effects including phototherapy, methotrexate, cyclosporine A, and mycophenolate mofetil. A number of newer drugs have been tried such as tofacitinib, apremilast, and ustekinumab with negative or modest effects, or significant side effects.
Therefore, there is an important unmet medical need for therapies in difficult-to-treat and severe atopic dermatitis. Some of the drugs in development will be discussed later in this article.
Galectin-3 Inhibition is a novel new mechanism that may be effective
Scientific studies show that there is a link between the galectin-3 protein and disease activity in atopic dermatitis. Galectin-3 protein is increased in the skin of patients, as well as animal models, of atopic dermatitis. Moreover, mice that lack the galectin-3 protein have much less skin disease when treated with a known trigger of allergic skin inflammation, induced by contact sensitization with an allergen (2). GR-MD-02, our galectin-3 inhibitor was used in patients because of these scientific data and the fact that there was a clinically significant effect in another inflammatory skin disease, psoriasis.
GR-MD-02’s potential for a clinically meaningful effect in refractory, severe atopic dermatitis
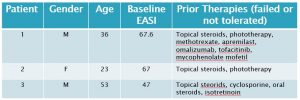
Three adult patients with severe atopic dermatitis were treated with GR-MD-02 by Dr. Simon Ritchie under an investigator-initiated protocol. All of these patients had failed a number of systemic therapies as shown in the table below, and would be classified as refractory which means their disease has not responded to treatment beyond topical steroids alone. The disease severity was determined using a standard, objective scoring system in common use for atopic dermatitis studies, the Eczema Area and Severity Index (EASI).
The disease was more severe in our three patients as indicated by the baseline EASI (median 67, mean 60), than in two recent phase 3 trials in atopic dermatitis, in which the median baseline EASI ranged from 29 to 31.8 for various groups (3). Moreover, the three patients in our study were refractory to systemic therapies, whereas the patients in these phase 3 studies only were required to fail topical steroid therapy. Other scores such as SCORAD were also used, but did not change the conclusions so only EASI is reported here for clarity.
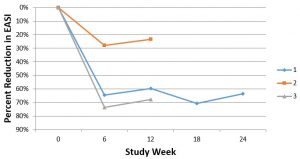
GR-MD-02 was given intravenously at a starting dose of 8 mg/kg every other week for 10 weeks (6 infusions) and then increased to 12 mg/kg every other week for an additional 7 infusions. All three patients experienced improvement in their atopic dermatitis, as shown below for the change in EASI scores. While patient 1 has completed the full 13 infusions, patients 2 and 3 have just started the higher dose of 12 mg/kg.
Patient 1, who completed the study, had a very significant reduction in disease activity and told Dr. Ritchie, “This is the best I have felt since I was diagnosed with atopic dermatitis 17 years ago”. In fact, the patient is so pleased with his therapy that it has been extended for another 24 weeks. It is also important to note that patient 1 had a significant improvement in the IGA scale (Investigator Global Assessment scale), starting with a score of 3 (0-4) and improving to a score of 1, an improvement that the FDA views as clinically significant in registration trials for atopic dermatitis. When patients 2 and 3 complete the additional therapy at the higher dose, we will report their final scores.
Patient 1’s skin was also visually improved with less thickness and scaling and re-growth of lateral eyebrows (lost from itching). However the cutaneous manifestations of atopic dermatitis are much more subtle and do not show well on photos (particularly in African American patients). This is the reason that photos are rarely, if ever, included in atopic dermatitis drug trial publications.
Importantly, there was no toxicity nor adverse events noted in the three patients.
How does GR-MD-02 treatment compare with other treatments in development?
GR-MD-02 seems to have had an important, clinically significant effect in this small open-label, investigator-initiated protocol. However, we caution that this is a very small sample of patients with no placebo treatment arm. In other documented trials, there has been a significant placebo response in atopic dermatitis (25% for a 50% improvement in EASI (called EASI-50) in dupilumab studies (3)), unlike in psoriasis trials where the placebo effect is usually less than 5%. On the other hand, all of these patients treated with GR-MD-02 had more severe baseline scores and refractory disease after multiple therapies, so it may be a more challenging group than those typical difficult-to-treat patients who have failed topical steroids only.
So, how does the effect we saw in this small study compare to other drugs in development? Dupilumab, a drug in development with successful phase 3 trials, averaged 64% of patients reaching an EASI-50 at week 16 of therapy (3). Two out of three of our patients reached an EASI-50 (reduction of EASI of 64% and 74%, respectively) at 6 weeks of therapy (3 doses of drug) which is a shorter period of time than with dupilumab. Moreover, as stated above, the baseline EASI score was more severe in our patients than in the phase 3 dupilumab studies and our patients had been on multiple systemic drugs, whereas those in the dupilumab studies had only to fail topical steroids.
Nemolizumab, another phase 3 drug asset, averaged 40% reduction in EASI at 12 weeks; patient 1 and 3 both exceeded this. Our patients 1 and 3 also faired favorably compared to tofacitinib, which has only been used in one small study, and there is not data on lebrikizumab which is currently in development.
Therefore, it appears that the effects on severe atopic dermatitis seen in our small, open label protocol, compares favorably with the other drugs currently in development.
What’s next?
Several arguments suggest that GR-MD-02 has potential as a therapy for severe atopic dermatitis:
- It represents a new and novel mechanism of galectin-3 inhibition in the disease
- We have seen clinically relevant, preliminary results of GR-MD-02 in three refractory patients with severe atopic dermatitis.
- There is a lack of effective therapy for these patients currently on the market
- GR-MD-02 has apparently similar magnitude of clinical effect levels compared to drugs in development
- GR-MD-02 has a very strong safety profile, with nearly 3,000 doses of GR-MD-02 delivered in multiple completed and ongoing clinical trials with no severe adverse effects attributed to the drug
- While intravenous administration is a potential drawback, it is encouraging that patient 1 asked to continue on therapy for an additional 6 months.
Phase 2, randomized, placebo-controlled trials with various doses and regimens of administration will be necessary to explore this potential. Planning for such a program is ongoing, as the company explores partnership and other options to finance such a program.
Reference List
- Arkwright PD, Motala C, Subramanian H, Spergel J, Schneider LC, Wollenberg A. Management of difficult-to-treat atopic dermatitis. J Allergy Clin Immunol Pract 2013 Mar;1(2):142-151.
- Saegusa J, Hsu DK, Chen HY, Yu L, Fermin A, Fung MA, et al. Galectin-3 is critical for the development of the allergic inflammatory response in a mouse model of atopic dermatitis. Am J Pathol 2009 Mar;174(3):922-931.
- Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N Engl J Med 2016 Dec 15;375(24):2335-2348.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including whether GR-MD-02 may be effective in the treatment of severe atopic dermatitis, psoriasis, and other skin disease. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2015, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
The post Galectin Inhibitor Therapy Effective in Severe Atopic Dermatitis (Eczema) appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post The Purpose of the CEO Perspectives Blog appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>First and foremost, the blog provides a mechanism for more detailed and economical communication with multiple groups and constituencies. It allows discussion of issues and expansion of information that is not appropriate for, or is constrained by, other outlets such as press releases and formal presentations. The posts are published to the Web, emailed to list of those whom have signed up for receiving company information, and distributed on social media including LinkedIn, Twitter, and Facebook. The goal is to inform and attract a broad readership.
As a company, we want to be as transparent about our activities as possible, and we want to use cutting-edge methods of communication to do so.
The content of the blog is intended to address direct programs of Galectin Therapeutics, general areas of importance for fatty liver disease (including liver fibrosis and cirrhosis), and the importance of galectin proteins in various diseases. In concert with regular press releases and meeting presentations, CEO Perspectives provides content that can be distributed to many different types of audiences. It is important to note, however, that press releases and SEC regulatory filings remain the only routes for communicating material company information. While I have at times published posts that complement press releases, the blog serves to supplement official communications from the company, not replace them.
The audiences that we intend to reach through the blog include current shareholders, prospective shareholders and investors, industry analysts, pharma and biotech companies, patients and advocacy groups, and the industry and popular press.
One of the main drivers behind CEO Perspectives is to help investors understand the tremendous value of what Galectin Therapeutics is doing in developing potential treatments for NASH cirrhosis and other diseases. The value of the company over the next year rests on success of our ongoing programs, which were outlined in various press releases and include:
- Data from the NASH-CX trial in patients with NASH cirrhosis which is completely enrolled and is scheduled to report top line data in December 2017. (see recent press release)
- The possibility of developing pharma partnerships for treating psoriasis and atopic dermatitis based on our exciting results of clinical effects in an exploratory clinical trial. (see recent press release)
- Combination immunotherapy trials being performed at Providence Cancer Center which will have interim data reported at Immunotherapeutics & Immunomonitoring Conference, to be held on February 6-7, 2017, in San Diego, California. (see recent press release)
For our shareholders and prospective investors, the posts are meant to supplement information in press releases and conference presentations. Sometimes they will be directly relevant to the value creation programs above, and sometimes not. I continue to value the feedback I get from investors about the blog, but other audiences are also important to the company.
For example, multiple industry analysts and pharma companies have found the blog useful in assessing our technology and programs. Many of the patients who are in our clinical trials or who have relevant disorders, as well as patient advocacy groups, have indicated the blog is helpful for their education.
The press is another important audience for the blog. The blog posts have proven useful to educate and inform reporters, which has led to boarder coverage of the company. Some examples of these results are the articles in the following industry and popular press outlets:
- Pharmacy Times—“Nonalcoholic Steatohepatitis Drug Pipeline Overview”
- HealthZette—“The New Liver Killer: Silent, Sneaky, Deadly”
- Drug Discovery & Development—“Why Non-Invasive Testing is Needed for Liver Fibrosis”
- Newsweek—“NASH is the 21st century’s looming public health threat”
- R&D Magazine—“Finding the “Holy Grail” Treatment for Fatty Livers”
The CEO Perspectives blog is meant to be informative for multiple audiences, to serve as content to expand the knowledge and understanding of Galectin Therapeutics’ programs, to bring more people to an understanding of the potential of our programs, and to educate people about the medical science that touches on our ultimate success. The path to an interest in the company can take multiple paths, and the blog helps to facilitate a number of them. It is not the only component of our outreach, but it has served as a useful mechanism for communication. I hope that all our audiences continue to find the posts useful.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including whether GR-MD-02 may be effective in the treatment of NASH and various other diseases. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2015, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Make a Comment or Ask a Question
[contact-form-7]The post The Purpose of the CEO Perspectives Blog appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Galectin-3, a Critical Link in the Cause of Diabetes appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>An important new research finding, published on November 3, 2016, in the scientific journal Cell, adds adult onset (type II) diabetes to the list of disorders with galectin-3 as a culprit (Li, P. et al. (2016) “Hematopoietic-derived galectin-3 causes cellular and systemic insulin resistance” Cell 167, 973-984). While this research was done in mice and therefore early, I believe it has potential important implications for human diabetes.
First, some background. Insulin is a peptide hormone made by cells in the pancreas secreted into the bloodstream to ultimately bind to the insulin receptors on many types of cells in the body. Insulin regulates energy utilization and metabolism in muscle, fat, and liver cells, which has the result of regulating the level of the blood sugar glucose.
Diabetes occurs when the blood glucose is not regulated effectively and is elevated above normal levels, which over time can lead to multiple complications. Type I diabetes, also called juvenile diabetes, occurs when there is an absolute deficiency of insulin due to reduced production by a damaged pancreas. Type II, or adult-onset diabetes, is associated with obesity and occurs when there is a relative deficiency of insulin due to resistance of target cells to the effects of insulin.
Insulin-resistance is the hallmark of type II diabetes. Because of this insulin resistance, insulin levels are actually increased in early stages of diabetes, but the pancreas loses the capacity to keep up production and insulin levels are eventually reduced in later stages of type II diabetes. One factor that seems to be involved in dysfunction of insulin receptors is the presence of inflammation, or immune cell infiltration, in fat tissue. This is where galectin-3 comes into play.
The study published in Cell shows that galectin-3 produced by macrophages (immune cell) binds to and inhibits the function of the insulin receptor, causing insulin resistance. The investigators demonstrated that removal of the galectin-3 gene or treatment with a galectin-3 inhibitor reduces insulin resistance in mice. In addition to revealing another factor involved in insulin resistance, this work demonstrates a direct link between an inflammatory cell protein, galectin-3, and dysfunction of the insulin receptor.
A beautiful aspect of science is how many findings from different perspectives can work into a tapestry that describes a much bigger picture. Insulin resistance in liver cells is a critical component of fatty liver disease, or non-alcoholic steatohepatitis (NASH), which is an enormous global medical problem. Our own research has shown that galectin-3 is increased in NASH livers. The new work on the effect of galectin-3 on insulin receptors adds another potential mechanism in the tapestry of the cause of NASH. Additionally, it provides another mechanism for the activity of our galectin-3 inhibitor GR-MD-02 in treating NASH as we have shown in animal studies and are studying in human clinical trials.
So, what does this all mean for diabetes and development of anti-galectin-3 drugs? If these studies are confirmed and extended to human disease, the galectin-3 link between inflammation and insulin resistance could represent an important new target for treatment of type II diabetes. At the present, there are no reported anti-galectin-3 drugs that are active by oral administration, which to my mind would be important for a diabetes indication. However, with this new finding, the search for such agents is likely to heat up. In this regard, Galectin Sciences, LLC, a wholly owned subsidiary of Galectin Therapeutics was formed in 2014 (see press release) for the purpose of discovering orally active galectin-3 inhibitors.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including whether GR-MD-02 may be effective in the treatment of NASH. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2015, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Make a Comment or Ask a Question
[contact-form-7]The post Galectin-3, a Critical Link in the Cause of Diabetes appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Results from the NASH-FX Study Underscore the Importance of Completing the NASH-CX Clinical Trial for Patients with NASH Cirrhosis appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The NASH-FX study was designed as a pilot study at a single site, involving only four months of treatment with GR-MD-02. This pilot study evolved from the results of a Phase 1 study in NASH patients with advanced fibrosis that had suggested FibroScan® measurements improved in three patients with just four doses of the study drug. The full report of this Phase 1 study will be published in the next few weeks in the peer-reviewed scientific journal, Alimentary Pharmacology and Therapeutics.
While most experts feel that liver fibrosis trials should have treatment phases for at least a year in duration, the results from the earlier Phase 1 NASH study provided a rationale for studying a larger group of patients with shorter therapy and exploring non-invasive technologies for assessing liver disease and fibrosis with a goal of using these technologies in later trials.
I want to give you a few details on the design of the NASH-FX study. The primary endpoint of NASH-FX was the LiverMultiScan (LMS), an approved magnetic resonance imaging test developed by Perspectum Diagnostics to assist in the diagnosis of liver disease. In NASH, the LMS is reported to measure the amount of fibrosis and inflammation. The LMS has low variability between scans in the same individual, and we used this low variability to calculate the number of patients we would need for the study to show a statistically significance difference between treatment groups with a power of 80%. In other words, the study was designed to have an 80% chance of showing a statistically significant difference in a 30 patient study, with 15 placebo and 15 GR-MD-02 patients. The power of the actual study, calculated after completion, was almost exactly an 80% chance to show an approximately 10% difference in LMS between placebo and treated groups. Therefore, the study design was adequate for the primary endpoint.
In contrast to LMS, the study was not powered for the secondary endpoints of liver stiffness, FibroScan and magnetic resonance elastography (MRE). The study would have required between 3 to 5 times as many patients to have an adequate power to show a difference with these tests. This is because the variability of these tests for repeated measurements is considerably greater than LMS.
However, we did not know before we conducted the study, nor did anyone else know, whether LMS correlated with the liver stiffness measurements of FibroScan and MRE. The NASH-FX study showed that there was poor correlation. Therefore, one cannot conclude that because there was no difference in LMS, that there would not be a difference in stiffness measurements, which have been shown in liver biopsy studies to correlate with fibrosis.
Although there was no apparent improvement in the three non-invasive tests for assessment of liver fibrosis in the four-month NASH-FX study, Dr. Stephen Harrison, a leading investigator in NASH and liver disease and the principal investigator of the NASH-FX study has pointed out that the inhibition of galectin-3 with GR-MD-02 remains promising for the treatment of NASH fibrosis. Dr. Harrison was especially encouraged that GR-MD-02 has demonstrated an improved clinical effect in moderate-to-severe psoriasis, suggesting the compound has activity in a human disease that can occur in association with NASH.
It remains critical that we complete the longer therapy NASH-CX clinical trial that has a much larger group of patients with NASH cirrhosis.
The NASH-CX trial is a one-year of treatment, multi-center trial in patients with NASH cirrhosis that is being conducted at 36 outstanding liver centers in the United States. The endpoints of the NASH-CX trial include invasive tests that are well-validated measures of liver disease severity. The primary endpoint is the change from baseline in the hepatic venous pressure gradient (HVPG), which measures the blood pressure in the liver and is well correlated with the clinical outcomes of patients.
Liver biopsy is an important secondary endpoint in the NASH-CX trial, which evaluates the stage of liver fibrosis and the amount of collagen, the primary component of fibrotic tissue. Finally, there are also non-invasive tests as secondary endpoints, including FibroScan and the 13C-methacetin breath test, which measures the metabolic function of the liver. These are important to correlate with the invasive tests because they may be useful in future trials and in management of patients.
I am pleased to report additional information on the status of this most important clinical trial as of October 10, 2016:
- The NASH-CX trial completed enrollment one month early with 162 total patients, exceeding the target of 156. This keeps us well on track for reporting of top-line results in December of 2017.
- The 162 patients were enrolled at 36 sites in the United States following the screening of 290 patients to obtain a population with well-compensated NASH cirrhosis (Child-Pugh-Turcotte Class A) with elevated portal pressure (HVPG ≥ 6 mmHg).
- In determining the number of patients to meet statistical requirements, we planned for the possibility that as many as 25% of the patients may drop out of the study during the treatment phase. However, we are pleased that only five patients of the 162 enrolled have dropped out of the study thus far. This low attrition rate highlights the importance, urgency, and need for patients suffering from NASH-cirrhosis to find an effective medical treatment.
- The low drop out trend also suggests that we will have a robust number of patients completing treatment for evaluation at the end of the trial. The trial was designed to have an 80% chance of demonstrating a 2 mmHg reduction in HVPG (i.e. 80% power) with 117 patients evaluated. Any number of patients above 117 will simply enhance the power of the study.
- At this point, 4 patients have completed the entire protocol and 70 patients have already completed six months of dosing.
- A total of 2,000 drug infusions (including placebo) have been given in this trial, representing 48% of the total number of infusions in the entire study. So we are quite pleased that this study is well along in its development.
The safety and tolerance of GR-MD-02 in all of the trials is most encouraging and supports our commitment to pursue the lead indication of NASH cirrhosis. In the NASH-FX study, GR-MD-02 was found to be safe and well-tolerated among the patient population with no serious adverse events related to the study medication. Over all of the clinical trials, including the patients in the NASH-CX trial, more than 1600 doses of GR-MD-02 have been administered without serious adverse effects related to the drug. This highlights the superior safety profile of the therapy in a patient population with advanced-stage disease, which is buttressed by the biological activity demonstrated in patients with moderate to severe plaque psoriasis.
Dr. Harrison, one of the two co-lead investigators in the NASH-CX trial, stated his belief that the inhibition of galectin-3 with GR-MD-02 remains a promising treatment for NASH fibrosis and that it is important to complete the NASH-CX trial.
Dr. Naga Chalasani, the other co-lead principal investigator of the NASH-CX trial, provided his assessment, stating:
“The results from the NASH-FX trial do not diminish the significance of the NASH-CX trial. Along with the safety and tolerability profile observed in the NASH-FX trial, the different patient population, much larger enrollment, rigorous study design and longer duration of therapy offer compelling rationale to complete the NASH-CX trial.”
As a company, Galectin Therapeutics’ attention has always been focused on completing the NASH-CX clinical trial and reporting results in a timely fashion.
With an outstanding safety profile, inhibition of galectin-3 with GR-MD-02 remains a potential treatment of NASH cirrhosis. Additionally, the longer therapy for one year, and endpoints that may serve as a surrogate for outcomes for registration trials in this patient population, provides us encouragement about our continuation of NASH-CX clinical trial.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including whether GR-MD-02 may be effective in the treatment of NASH. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2015, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Make a Comment or Ask a Question
[contact-form-7]The post Results from the NASH-FX Study Underscore the Importance of Completing the NASH-CX Clinical Trial for Patients with NASH Cirrhosis appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Fatty Liver is an Enormous Global Problem appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>Younossi and his colleagues collected data on over 8.5 million people from 22 countries, using a method called meta-analysis which allows combining data from multiple studies. (I have described this method in a previous Perspective.) Rinella and Charlton nicely summarized the main findings of this very important study in their editorial.
Before I describe the main findings, I must define some terms. Non-alcoholic fatty liver disease, or NAFLD, refers to abnormal accumulation of fat in liver cells. In some individuals, NAFLD progresses to NASH, or non-alcoholic steatohepatitis. In NASH, the presence of inflammation and dying liver cells is added to the accumulation of fat in liver cells. Some individuals with NASH have progressive fibrosis, or scarring of the liver, which may ultimately lead to cirrhosis, complications of cirrhosis, the need for liver transplant, and death.
The first conclusion of the study is that approximately one in four people in the world are affected by NAFLD. There are geographical differences in the rates of NAFLD across the world, as shown in the figure below published in the editorial (the numbers represent the percent of people in that region who have NAFLD).
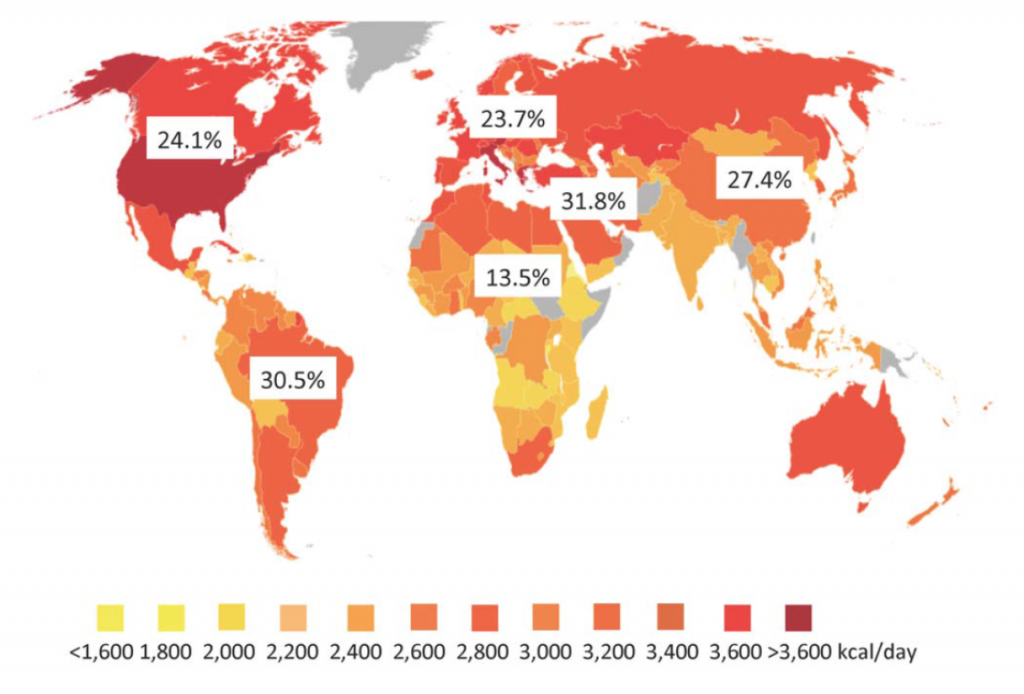
The color coding on this graph relates to the average caloric intake in the individual countries. While it is well known that obesity is associated with NAFLD, the relatively poor correlation with caloric intake with NAFLD rates suggests there are other factors involved. Some of these factors may include genetics (important in some regions), the type of food ingested, the composition of bacteria in the gut, and others.
The second major point taken from this study is that progression of fibrosis in NAFLD is slow and unusual. The editorial authors include an instructive figure to demonstrate this point. Out of 100 patients with NAFLD, only 5 will develop cirrhosis (the end stage of scarring) and only 1-2 patients will die as a result of that cirrhosis or require a liver transplant.
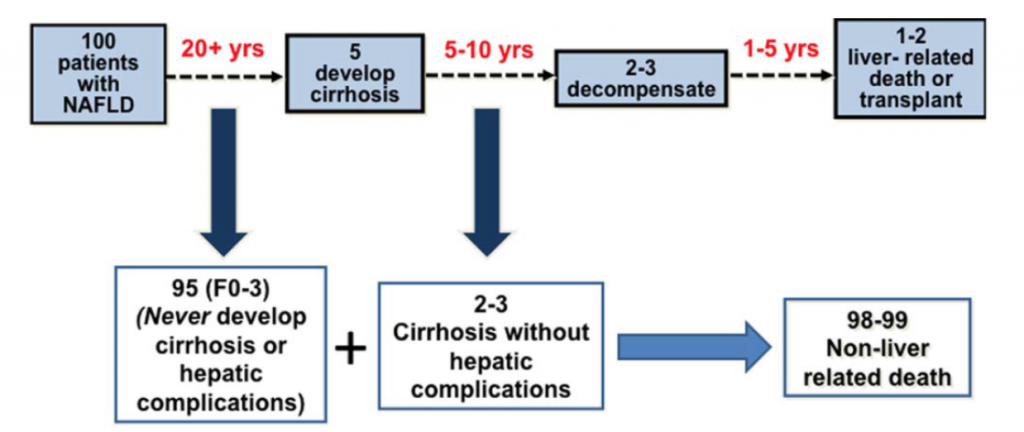
On face value, this seems like a small number of patients dying of their liver disease. However, when one considers the large number of people with NAFLD, those with cirrhosis represent a very significant problem. As stated by the authors, “Despite the low frequency of liver related mortality, the staggering denominator of over 1 billion adults with NAFLD coupled with a life-time risk of ~2% for liver-related mortality will eventually translate into ~20,000,000 liver-related deaths among patients currently alive with NAFLD.”
The final point is that NAFLD is, or will soon become, the most common cause of liver disease and liver-related death globally. The most important target for dealing with this enormous health problem is related to life-style changes to maintain a healthy liver, as I described in a previous Perspective. However, since there are currently no medicines available for this disease, we must also focus on those 20,000,000 people who are destined to die of NASH cirrhosis. This is the target for our Phase 2 clinical trial, NASH-CX, using our drug candidate GR-MD-02.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. For a discussion of factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2015, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Reference List
1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016 Jul;64(1):73-84.
2. Rinella M, Charlton M. The globalization of nonalcoholic fatty liver disease: Prevalence and impact on world health. Hepatology 2016 Jul;64(1):19-22.
Make a Comment or Ask a Question
[contact-form-7]The post Fatty Liver is an Enormous Global Problem appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Can Drinking Coffee Cure NASH or Liver Fibrosis? appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The short answer is, “Yes, drinking coffee does seem to reduce your chances of developing cirrhosis.” However, it’s not that simple so I wanted to dive into this news in a little more depth.
First, the relationship between coffee and cirrhosis is a topic that’s been examined in a number of different studies. For example, Stephen Harrison of Brooke Army Medical Center, one of the principal investigators in our NASH-FX trial and co-lead investigator in our NASH-CX trial, published a paper last year that showed an inverse relationship between coffee consumption and NASH activity, i.e., the more coffee you drank, the milder your NASH.
This most recent study was a meta-analysis of many different studies – a study of studies, if you will. These kinds of studies are very common, having been used to establish all things we know about smoking and lung cancer, for example, or cholesterol and heart disease. In fact, one of the studies used in the meta-analysis was from a heart study group; once you have characterized a large set of individuals for one purpose, you can look at the data generated by that study for other things as well.
There’s a formal, scientific process for undertaking this sort of research that involves culling the entire literature to find every single paper that examines a particular issue – in this case cirrhosis and coffee consumption – and then using a list of criteria to choose the best papers for combining in a meta-analysis. Nine studies met the criteria for this particular meta-analysis, resulting in a total studied population of more than 430,000 individuals. This is a very large sample size, which allows for greater confidence in the findings.
The evidence seems clear that coffee consumption is associated with lower rates of the development of cirrhosis. The study established a dose-response relationship based on the number of cups of coffee consumed per day. To summarize the findings in everyday language, if you increase your consumption of coffee by two cups per day, you decrease your likelihood of developing cirrhosis by half, which is a pretty profound effect.
However, there are many things this study doesn’t tell us: How long do you have to drink coffee to have this effect? At what age do you have to start? If you have liver disease, will drinking coffee help you? If you are out drinking alcohol, will ending the night with a couple of cups of coffee protect your liver? Does the brand or strength of the coffee make a difference? No, that’s not what was studied here (despite what the headline in CNN’s story might imply). It was simply an examination of how many people in this population have cirrhosis – or not –and how much coffee they say they drink. The relationship seems pretty strong, but this research doesn’t answer any of the other questions that people might have.
From a biochemical perspective it makes sense that coffee might have this effect. Many people tend to think of coffee as a dark liquid that has caffeine, but coffee is actually very complex, with hundreds of biologically active compounds. The vast majority of these compounds are taken up by the liver and are metabolized, just as the liver does with anything we eat and many drugs that we take.
This effect doesn’t extend to other caffeine-containing drinks. It appears to be the coffee that provides beneficial effects on the liver, not the caffeine. There have been studies comparing caffeinated with decaffeinated coffee, thereby isolating the effects of caffeine, and it appears likely that it’s not the caffeine that is the important factor. You can’t drink lots of Red Bull® and get the same effect.
What does this mean for patients and for physicians? To me, none of this precludes a common sense approach to liver health, as I published in a previous Perspective. However, coffee does seem to protect against liver disease, and there are other benefits that have been identified for coffee as well, including a reduction in all-cause mortality, neurological disease and some cancers, as recently reported for colon cancer. These benefits are balanced by some potential negative things about coffee. There are far fewer studies that suggest that coffee is bad for you, but there are some. For instance, there’s been a reported association with lung cancer. However, that effect is completely over-shadowed by tobacco use; people often smoke cigarettes while they’re drinking coffee, so it’s unclear that the coffee is to blame.
It’s possible we’ll never truly understand what it is about coffee that is responsible for this effect. There are people conducting experimental studies in animals and in cell lines, looking at the individual components of coffee in an attempt to determine which are having an effect on the fibrotic process. At some point, researchers may identify “compound X” within coffee, but we are a long way from turning this connection into something that would play an active role in the treatment of fibrosis and cirrhosis.
On balance, as a physician looking at these data, I think it makes sense to advise people who enjoy coffee to drink coffee. If coffee doesn’t bother you, and you’d like to have two or three cups a day, go ahead. It may benefit your liver, and there’s nothing like a good cup of coffee. That said, I wouldn’t think it right for a physician to start writing prescriptions for drinking coffee.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. For a discussion of factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2015, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Make a Comment or Ask a Question
[contact-form-7]The post Can Drinking Coffee Cure NASH or Liver Fibrosis? appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Love Your Liver: A Prescription for Liver Health appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>I am often asked how one can maintain a healthy liver and avoid the complications that can happen with chronic fatty liver disease. It has also recently been shown that fatty liver disease is an independent risk factor for cardiovascular disease, providing everyone with another important reason to maintain a healthy liver.
Liver health should be as important to everyone as heart health. However, how do you go about ensuring that your liver is healthy? I have listed below my suggested steps to maintain a healthy liver.
Maintain a healthy weight
There is a clear connection between body weight and the risk of NASH, including an increase in organ fat in apparently lean people (see here). If you are overweight, losing as little as 10% of your body weight can result in a much healthier liver (see here).
Follow a healthy diet
It is not my intention to provide specific dietary advice, but your liver diet should be preferably high in lean protein, low in carbohydrate like starch, low in added sugar, and limited high fructose corn syrup.
Exercise
Activity appears to improve fatty liver disease and help keep your liver healthy even if you don’t lose weight. Studies show resistance exercise is as beneficial as aerobic exercise.
Avoid excessive alcohol
Know the exact alcohol content of what you are drinking and what constitutes a “drink.” Men should drink a maximum of two drinks a day and women should limit themselves to one drink a day.
Avoid certain nutritional supplements
Certain nutritional supplements and herbal remedies can damage the liver. Talk to your doctor or read on-line about all supplements you are considering. For example, even too much iron or vitamin A can be harmful to your liver.
No illicit drug use
This may seem obvious, but you must avoid the use of drugs that expose you to blood from others, which put you at risk for hepatitis.
Discuss liver health with your doctor
Ask your primary care doctor, “How’s my liver?” You will often have gotten routine blood tests that include liver enzymes that may provide an indication of liver problems. Also, always ask whether there are potential liver effects from drugs, over-the-counter medicines, nutritional supplements and vitamins you may be taking.
These all seem like simple steps, but if everyone were following them we would not have the epidemic of fatty liver disease and other liver diseases we have today. Consider incorporating these steps in your lifestyle today.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. For a discussion of factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2015, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Make a Comment or Ask a Question
[contact-form-7]The post Love Your Liver: A Prescription for Liver Health appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Photographs of Improvement in Moderate-To-Severe Plaque Psoriasis appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>Patients treated with GR-MD-02 experienced significant clinical improvement
As described in the press release, each of the four patients who received six doses of GR-MD-02 over 12 weeks of therapy reported improvement in their psoriasis symptoms. Moreover, three patients had significant, and one had modest, improvement in the Psoriasis Area Severity Index (PASI), which is an objective and standardized means of evaluating disease response (see table below).
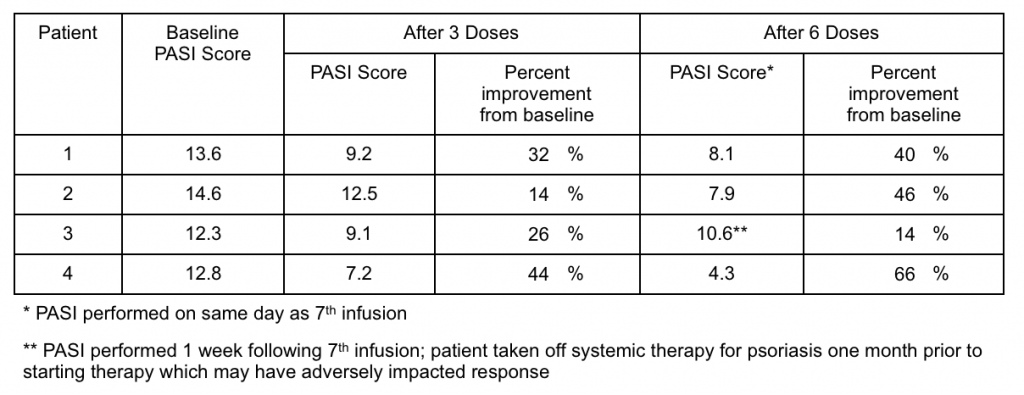
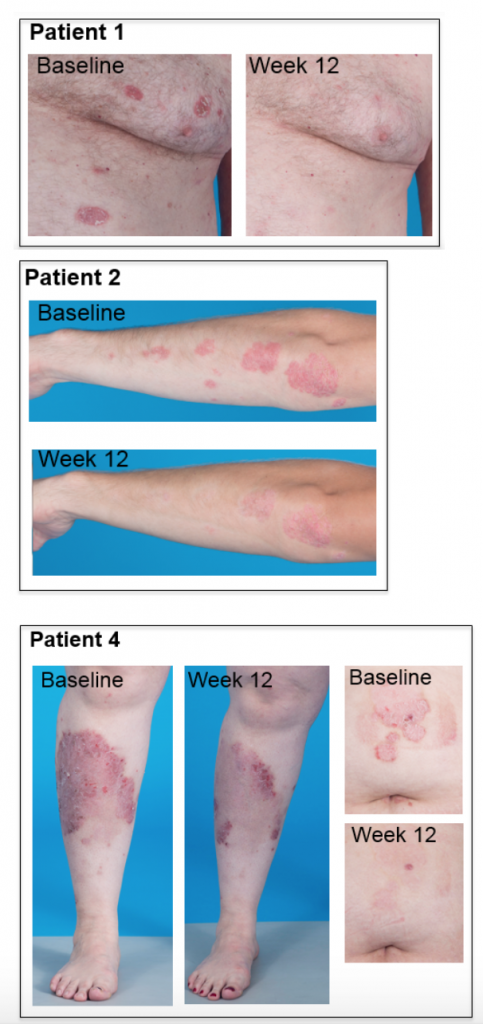
In addition to these quantitative results, photographs provide vivid evidence of response to therapy (below). Each patient had over 70 pictures taken at each photography session, so this represents only a small sample and not a full representation of their whole body disease. These photographs provide evidence of disease improvement in areas of significant improvement.
Patient 1 had complete clearing of multiple chest lesions. Patient 2 had near complete resolution of arm and elbow lesions. Patient 4, the one with the greatest reduction in PASI score, had nearly complete resolution of an abdominal lesion and remarkable improvement of very severe leg lesions. Most of the disease on Patient 4’s leg is actually completely resolved with only some skin hyperpigmentation, which generally takes months to slowly fade.
What does this mean for our drug GR-MD-02?
It is uncommon for patients with moderate-to-severe plaque psoriasis to spontaneously improve without treatment, so the improvements we have seen in this trial are due to GR-MD-02. Furthermore, these improvements are clinically significant for these patients, as all of them suffered from moderate-to-severe psoriasis for years (between 6 and 40 years) prior to entering the trial. Therefore, it is our belief that GR-MD-02 had a positive effect on psoriasis in these patients.
We believe this is the first definitive clinical effect of GR-MD-02 to be shown in patients. While all diseases are not the same in terms of etiology, these results support our optimism for GR-MD-02 to affect other disease processes that the company is investigating.
How do our results with GR-MD-02 compare with other available psoriasis therapies?
Beyond the fact that GR-MD-02 has a biological effect on psoriasis in patients, we need to consider the possibility that GR-MD-02 could be additive to the medical armamentarium for treating psoriasis. There are multiple commercial and development-stage drugs for the treatment of moderate-to-severe plaque psoriasis, including biological agents that would constitute the direct competition for GR-MD-02.
There is an established FDA regulatory pathway for drug approval in moderate-to-severe plaque psoriasis that all the recent marketed biological drugs have followed. PASI is universally used in registration clinical trials (also referred to as phase 3 or pivotal clinical trials) as an objective measurement of drug response. All registration clinical trials assess the number of patients who achieve a 75% improvement in PASI (called PASI-75) at various times following initiation of therapy. For currently approved drugs, the percentage of patients who achieve PASI-75 at between 12 and 24 weeks of therapy ranges from 54% to as high as 90% in some studies.
At the time of this interim analysis of our trial, none of the four patients had reached a PASI-75, which is why we have taken the next steps described below.
What are the next steps?
We have extended therapy in the study with the current dose of GR-MD-02 for a full 24 weeks, or 13 infusions, to determine whether improvement at this dose continues and reaches PASI-75. Extension beyond 24 weeks of therapy to reach PASI-75 would not be competitive from an efficacy standpoint among the currently marketed biological agents for moderate-to-severe plaque psoriasis. However, in general, approval of drugs is a balance between efficacy and safety and most of the drugs on the market have serious side effect profiles. We are now attempting to enroll the full cohort of 10 patients in this trial, with all receiving 24 weeks of therapy. We will continue to report interim data from this trial.
This interim analysis of four patients has given us great encouragement on the biological effect of GR-MD-02 in a human disease and we look forward to reporting results later this year on this trial and our trial with GR-MD-02 in patients with NASH and advanced (stage 3) fibrosis (the NASH-FX trial).
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including whether GR-MD-02 may be effective in the treatment of psoriasis. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2015, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Make a Comment or Ask a Question
[contact-form-7]The post Photographs of Improvement in Moderate-To-Severe Plaque Psoriasis appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Pulmonary Arterial Hypertension is Associated with Elevated Levels of Galectin-3 and a Potential Therapeutic Target for GR-MD-02 appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>Galectin Therapeutics is collaborating with a number of academic groups to evaluate cardiovascular diseases that might be amenable to treatment with our galectin inhibitor drugs. In this article, I discuss the results obtained by the outstanding investigators at the Vascular Biology Center and the Department of Pharmacology and Toxicology at Augusta University (formerly known as Georgia Regents University), which were recently presented at the 2016 American Thoracic Society International Conference and highlighted in a recent press article.
What is pulmonary arterial hypertension (PAH)?
Pulmonary hypertension (PH) is high blood pressure in the main arteries that supply blood to the lungs, caused by the narrowing and constriction of blood vessels in the lungs themselves. This elevated blood pressure increases the work required of the heart’s right ventricle to pump blood into the lung, which eventually culminates in failure of the right ventricle, circulatory collapse and death. The causes of PH have been categorized into five groups. Group 1, also called pulmonary arterial hypertension (PAH), includes idiopathic and hereditary PAH, as well as multiple disorders that affect the small arterioles (blood vessels) in the lung. The remaining four groups are secondary to other disease including left heart failure (Group 2), chronic lung disease (Group 3), chronic pulmonary embolism (Group 4) or multifactorial mechanisms (Group 5).
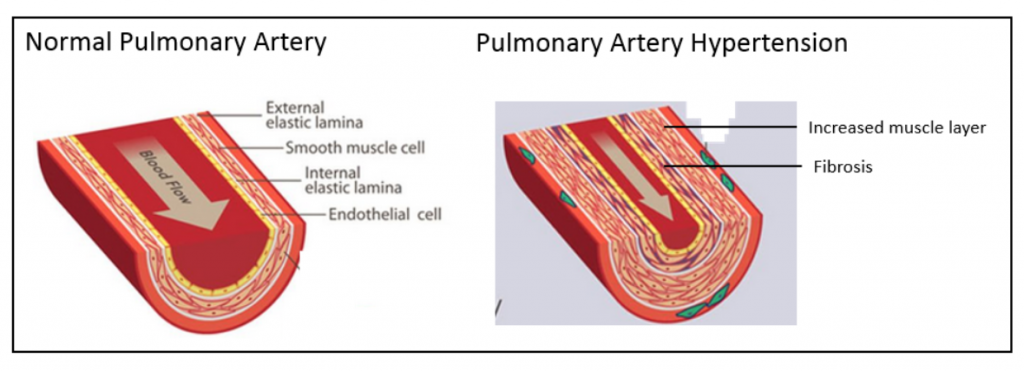
The figure below on the left shows a normal pulmonary artery with the various layers of cells in the wall, including the external elastic lamina on the outside of the artery, the smooth muscle cell layer, the internal elastic lamina and the endothelial cell lining on the inside. As depicted in the figure below on the right, in PAH there is a marked increase in the thickness of the smooth muscle cell layer as well as fibrosis. This narrows the lumen of the artery and restricts blood flow.
One of the more pressing needs is for new PH therapies in Group 1 disorders, particularly idiopathic PAH. Although there are a number of vasodilator drugs that are approved for the treatment of PAH, additional therapies are needed to address the structural narrowing of pulmonary arteries..
Galectin-3 protein is markedly elevated in human and experimental pulmonary arterial hypertension
The investigators at Augusta University experimented with three animal models of PAH using chemical treatments and/or low oxygen environments. These models are well established to cause narrowing of the pulmonary blood vessels leading to stress and failure of the right ventricle, meaning they mimic what happens in human PAH. The investigators found that the galectin-3 protein was markedly higher in the pulmonary arteries of diseased animals and the degree of increased blood pressure correlated with the level of galectin-3 elevation.
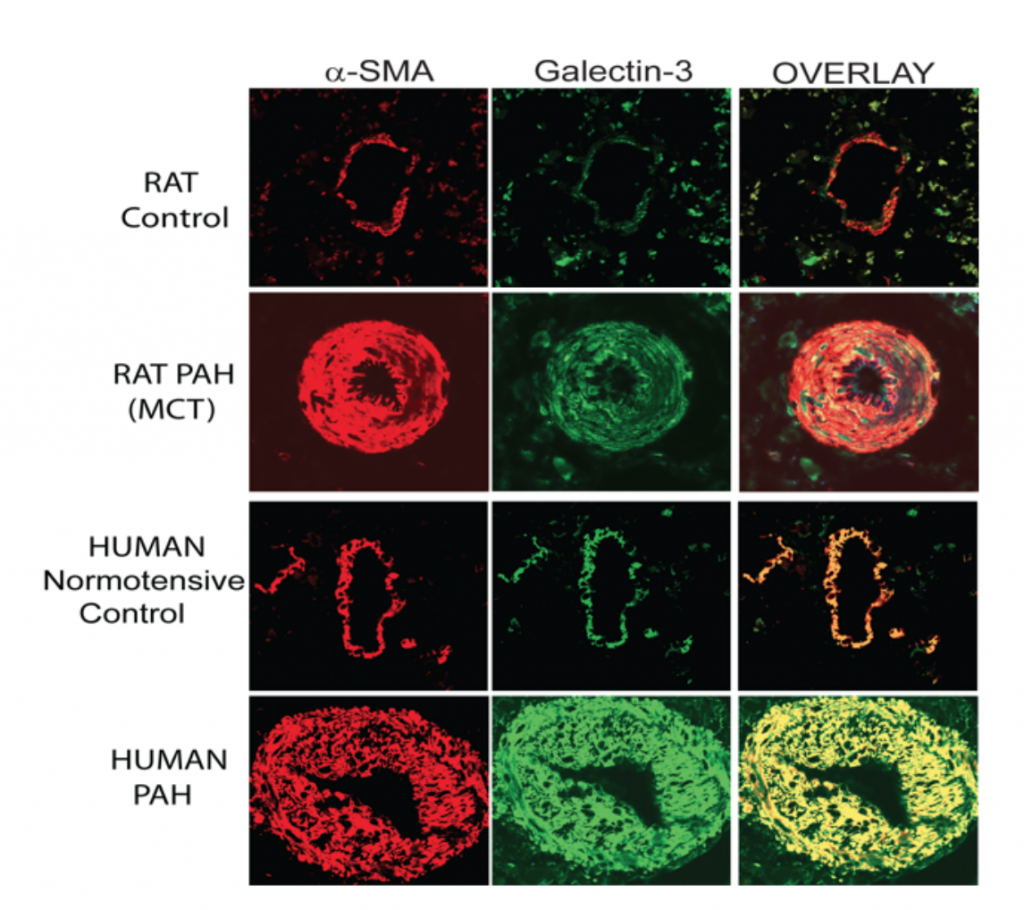
Since a picture is worth a thousand words, I have reproduced below images of the microscopic assessment of galectin-3 in pulmonary arteries in one rat model with monocrotaline treatment (MCT), as compared with the human disease.
The red stain is for the protein alpha smooth muscle actin (α-SMA), which stains smooth muscle cells in the artery, and the green stain is for the galectin-3 protein; the overlay combines the two images and shows that galectin-3 is predominantly in smooth muscle cells. In both the normal rat and the human pulmonary arteries, a thin layer of smooth muscle cells that contain galectin-3 is visible. In the rats and humans with PAH there is a marked increase in thickness of the muscle cells that surround the artery associated with a marked increase in galectin-3.
GR-MD-02 is effective in experimental pulmonary hypertension
The investigators next tested whether our galectin-3 inhibitors were able to change the course of experimental PAH using the model in which the rats were given a single injection of MCT to induce pulmonary hypertension. Two of our galectin inhibitors were used as treatments: GR-MD-02, which is currently in clinical trials for NASH, psoriasis, and cancer, and GM-CT-01.
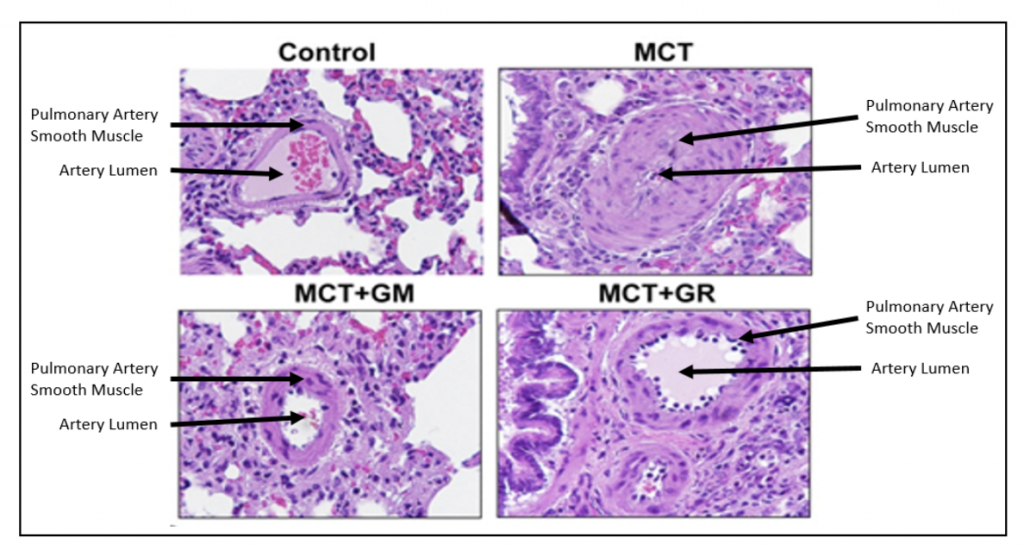
As shown in the figure below, the control (normal) rat pulmonary artery has a thin layer of smooth muscle cells surrounding it, whereas the animals treated with MCT have a markedly thickened smooth muscle layer that nearly blocks the lumen of the artery. Treatment of MCT animals with either GR-MD-02 or GM-CT-01 resulted in significantly fewer smooth muscle cells and a much larger artery lumen.
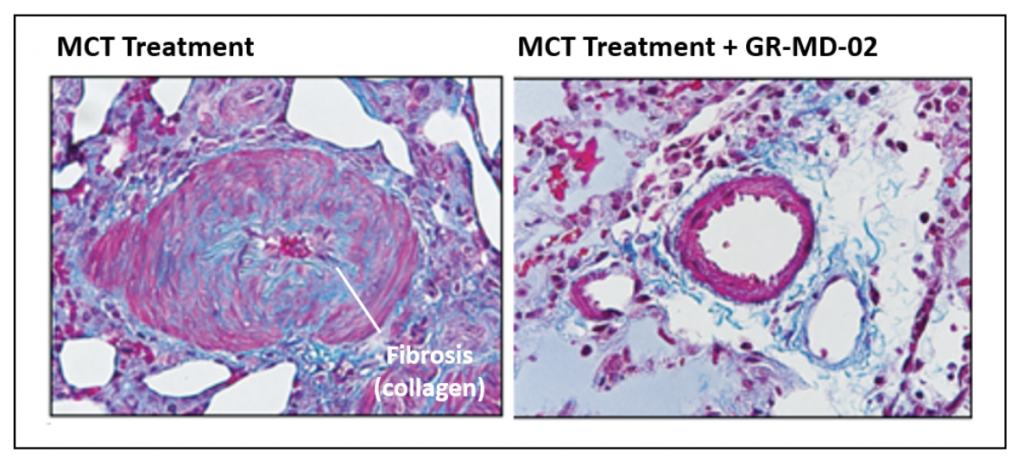
Below are images of pulmonary arteries stained to show fibrosis, mostly the protein collagen, which is identified by the light blue material. In the MCT treated animals, there is a significant amount of fibrosis associated with the smooth muscle cells in the pulmonary artery. This fibrosis is virtually eliminated in the artery wall following treatment with GR-MD-02, which provides a link to data showing improvement in fibrosis in other models of disease in liver, lung and kidney.
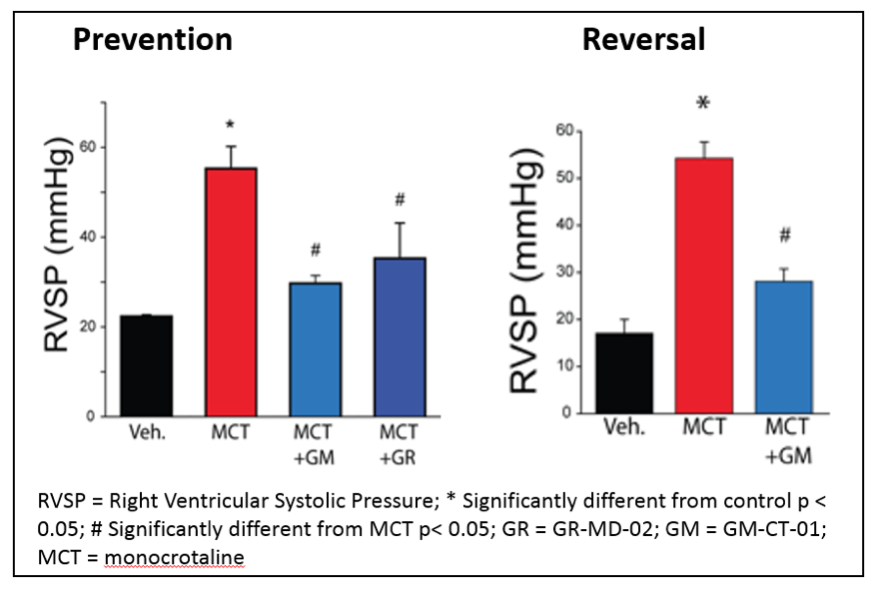
Treatment with either GR-MD-02 or GM-CT-01 also resulted in functional improvement in the PAH rats as indicated by reduced right ventricular systolic pressure (RVSP), as shown below. This improvement was seen whether the treatment was started immediately after the MCT injection (Prevention) or three weeks after the MCT injection (Reversal).
Prospects for human therapy
The results from these animal studies suggest that exploration of anti-galectin therapies, and specifically with GR-MD-02, might be a new therapeutic approach for these seriously ill patients. Currently there are approved therapies that help dilate constricted pulmonary arteries, but there are no therapies that effectively change the structure of the arteries or reverse the long-term course of the disease. A drug able to reverse the arterial smooth muscle and fibrosis findings in PAH might play an important role in treating these patients.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including whether GR-MD-02 and GM-CT-01 may be effective in the treatment of pulmonary arterial hypertension. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2015, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Make a Comment or Ask a Question
[contact-form-7]The post Pulmonary Arterial Hypertension is Associated with Elevated Levels of Galectin-3 and a Potential Therapeutic Target for GR-MD-02 appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post How Will We Interpret Results from the GR-MD- 02 Psoriasis Trial? appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>Last September, Galectin Therapeutics began a Phase 2a open-label clinical trial with GR-MD-02, our galectin-3 inhibitor drug, in patients with moderate-to-severe plaque psoriasis. We initiated this trial because a patient in our Phase 1 trial with GR-MD-02 in NASH had a remarkable and sustained remission of her psoriasis following treatment, as described in a previous CEO Perspective (here). Since then, I have been asked on numerous occasions how we will interpret the results of this trial.
What are the psoriasis trial endpoints?
Multiple drugs have been approved for the treatment of psoriasis, so there is an established FDA regulatory pathway to approval. It is well accepted that assessment of drug efficacy in psoriasis clinical trials is best performed using the Psoriasis Area and Severity Index, or PASI. PASI is used both to measure the severity of psoriasis as well as to measure the effect of treatment. While this index score is not generally used in the clinical practices of dermatology, it is universally used in clinical trials as an objective measurement of drug response.
PASI is an objective set of measurements that are conducted by a physician to assess the area of skin involvement and the severity of skin lesions in patients with psoriasis. The figure below, taken from a review article written by experts in the field, outlines the approach to the measurement (1).
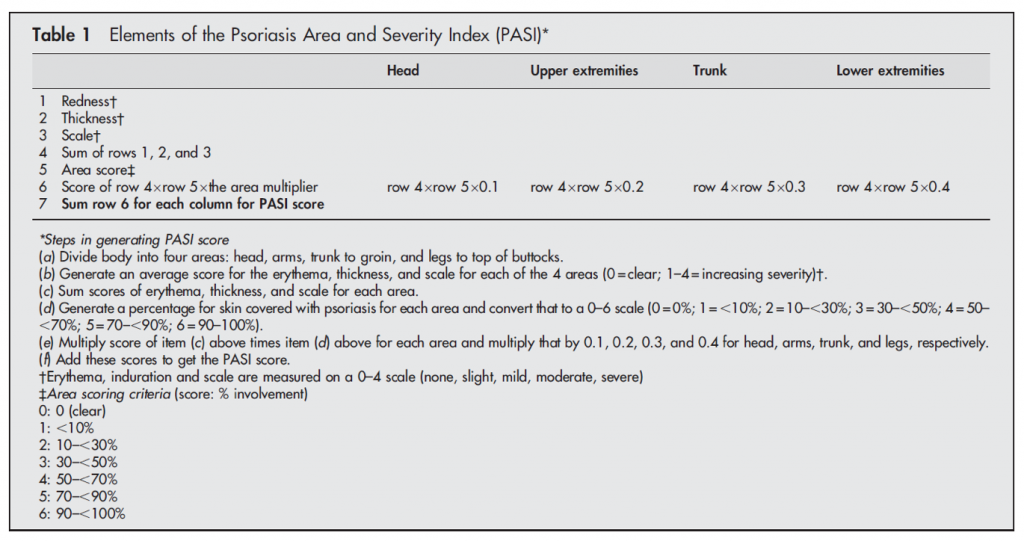
Therefore, the primary endpoint in our psoriasis trial will focus on improvement in PASI following treatment with GR-MD-02. We will evaluate the number of patients who have various percentage improvements in PASI. We will also record the durability of response to treatment for up to one year, should there be a beneficial effect. Finally, we are monitoring the incidence of adverse events and vital sign and laboratory abnormalities, if any, during study treatment.
What constitutes a meaningful effect?
Moderate psoriasis is generally defined as a PASI of ≥10. In our trial, as is typical for clinical trials in moderate-to-severe psoriasis, patients who enroll in the trial are required to have at least 10% of their body area affected by psoriasis and a PASI of ≥12, parameters which were agreed to with the FDA. Moreover, the patients will have the diagnosis of psoriasis confirmed by a skin biopsy. Therefore, patients in the trial will have significant disease and are similar to those who have been enrolled in the trials of approved drugs for psoriasis.
Once an individual has established psoriasis, it generally does not spontaneously dissipate or improve. As published in the medical literature, it has been suggested that clinically significant improvements in psoriasis are clearly obtained when PASI is lowered by 50% of baseline values, i.e., a PASI 50 response (2). However, the accepted clinical trial endpoint for drug registration is PASI 75, meaning a 75% reduction in psoriasis versus baseline. However, since this is an exploratory trial in which we do not have any information on optimal dosing, we will be looking for any objective response.
What will this trial tell us and what might be the next steps?
First and most important, our exploratory psoriasis clinical trial will either confirm the effect on psoriasis seen in the one Phase 1 patient, or show that her remission was unrelated to our drug. Should a significant proportion of patients in the trial respond to GR-MD-02 with an improvement in their psoriasis, such a finding would represent the first human disease response to GR-MD-02. That would be an important finding with potential implications for activity in our main therapeutic program for NASH, a disease where there is a higher incidence of psoriasis.
Second, this trial may indicate whether GR-MD-02 is a viable candidate to advance as a therapy for psoriasis. There are a number of biologic agents that have been approved in recent years for moderate-to-severe psoriasis, and GR-MD-02 would need to be competitive with those drugs. In my view, based on the efficacy of approved drugs, GR-MD-02 would need to demonstrate a PASI 75 in at least 50% of treated patients to consider full clinical development. If it does have such a degree of efficacy, it might be considered as a potential drug therapy on the basis of its lower cost to manufacture than existing drugs, a potentially better safety profile or the duration of effect after stopping treatment.
While each of these potential benefits would need to be studied in detail, the first step is to determine whether the drug has an effect on the disease, which is the primary goal of this study.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including whether GR-MD-02 may be effective in the treatment of psoriasis. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2015, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Reference List
1. Feldman SR, Krueger GG. Psoriasis assessment tools in clinical trials. Ann Rheum Dis 2005 Mar;64 Suppl 2:ii65-ii68.
2. Carlin CS, Feldman SR, Krueger JG, Menter A, Krueger GG. A 50% reduction in the Psoriasis Area and Severity Index (PASI 50) is a clinically significant endpoint in the assessment of psoriasis. J Am Acad Dermatol 2004 Jun;50(6):859-866.
Make a Comment or Ask a Question
[contact-form-7]The post How Will We Interpret Results from the GR-MD- 02 Psoriasis Trial? appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>