Guest Author
I welcome as a co-author to this Perspective, Dr. Simon Ritchie, our lead investigator in skin disease and staff dermatologist, at San Antonio Military Health System, Fort Sam Houston, TX. Dr. Ritchie conducted the clinical study in moderate-to-severe plaque psoriasis and will present the promising results of the effect of our galectin inhibitor GR-MD-02 in psoriasis at the Maui Derm meeting March 20-23. The progression of results in the treatment of psoriasis has been previously released on March 6, 2016, May 16, 2016, and November 10, 2016.
What is atopic dermatitis (Eczema)
Atopic dermatitis, commonly called eczema, is a chronic inflammatory condition of the skin of caused by multiple factors that usually arises in early childhood, often in infancy. While it usually resolves by early teenage years, approximately 5-10% of patients have the disease extend into adulthood. As an allergic type of disorder, it is associated with asthma and seasonal allergies, which often occur in the same patient called the “atopic triad”. Atopic dermatitis is the itch that becomes a rash. Classic symptoms are itching and burning of the skin, resulting in thickening of the skin in response to the scratching. In some adults, it can be severe with debilitating itching, inability to sleep, and social stigmatization due to skin damage and thickening, often on the face.
How big a problem is atopic dermatitis?
Surveys suggest that up to 18% of the population have atopic dermatitis, up to 37% of those people seek medical care, and over 70% of those seeking care have mild disease that is handled by primary care physicians. Of those patients who seek medical care, approximately 20% have moderate and 2% have severe disease, which is generally referred to specialists. The national estimated cost of treatment is as high as $3.8 billion, as of 2012 (1).
No new therapies for difficult-to-treat atopic dermatitis in 30 years
Mild atopic dermatitis can be treated with skin moisturizers and topical steroid preparations, but there are fewer options for those that have more severe disease and do not respond. The only FDA approved systemic therapy is oral steroids, but multiple other drugs and approaches are often tried in clinical practice with limited efficacy and significant side effects including phototherapy, methotrexate, cyclosporine A, and mycophenolate mofetil. A number of newer drugs have been tried such as tofacitinib, apremilast, and ustekinumab with negative or modest effects, or significant side effects.
Therefore, there is an important unmet medical need for therapies in difficult-to-treat and severe atopic dermatitis. Some of the drugs in development will be discussed later in this article.
Galectin-3 Inhibition is a novel new mechanism that may be effective
Scientific studies show that there is a link between the galectin-3 protein and disease activity in atopic dermatitis. Galectin-3 protein is increased in the skin of patients, as well as animal models, of atopic dermatitis. Moreover, mice that lack the galectin-3 protein have much less skin disease when treated with a known trigger of allergic skin inflammation, induced by contact sensitization with an allergen (2). GR-MD-02, our galectin-3 inhibitor was used in patients because of these scientific data and the fact that there was a clinically significant effect in another inflammatory skin disease, psoriasis.
GR-MD-02’s potential for a clinically meaningful effect in refractory, severe atopic dermatitis
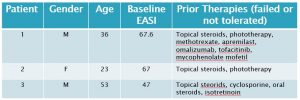
Three adult patients with severe atopic dermatitis were treated with GR-MD-02 by Dr. Simon Ritchie under an investigator-initiated protocol. All of these patients had failed a number of systemic therapies as shown in the table below, and would be classified as refractory which means their disease has not responded to treatment beyond topical steroids alone. The disease severity was determined using a standard, objective scoring system in common use for atopic dermatitis studies, the Eczema Area and Severity Index (EASI).
The disease was more severe in our three patients as indicated by the baseline EASI (median 67, mean 60), than in two recent phase 3 trials in atopic dermatitis, in which the median baseline EASI ranged from 29 to 31.8 for various groups (3). Moreover, the three patients in our study were refractory to systemic therapies, whereas the patients in these phase 3 studies only were required to fail topical steroid therapy. Other scores such as SCORAD were also used, but did not change the conclusions so only EASI is reported here for clarity.
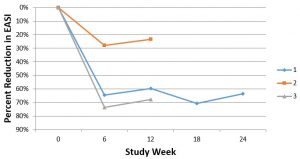
GR-MD-02 was given intravenously at a starting dose of 8 mg/kg every other week for 10 weeks (6 infusions) and then increased to 12 mg/kg every other week for an additional 7 infusions. All three patients experienced improvement in their atopic dermatitis, as shown below for the change in EASI scores. While patient 1 has completed the full 13 infusions, patients 2 and 3 have just started the higher dose of 12 mg/kg.
Patient 1, who completed the study, had a very significant reduction in disease activity and told Dr. Ritchie, “This is the best I have felt since I was diagnosed with atopic dermatitis 17 years ago”. In fact, the patient is so pleased with his therapy that it has been extended for another 24 weeks. It is also important to note that patient 1 had a significant improvement in the IGA scale (Investigator Global Assessment scale), starting with a score of 3 (0-4) and improving to a score of 1, an improvement that the FDA views as clinically significant in registration trials for atopic dermatitis. When patients 2 and 3 complete the additional therapy at the higher dose, we will report their final scores.
Patient 1’s skin was also visually improved with less thickness and scaling and re-growth of lateral eyebrows (lost from itching). However the cutaneous manifestations of atopic dermatitis are much more subtle and do not show well on photos (particularly in African American patients). This is the reason that photos are rarely, if ever, included in atopic dermatitis drug trial publications.
Importantly, there was no toxicity nor adverse events noted in the three patients.
How does GR-MD-02 treatment compare with other treatments in development?
GR-MD-02 seems to have had an important, clinically significant effect in this small open-label, investigator-initiated protocol. However, we caution that this is a very small sample of patients with no placebo treatment arm. In other documented trials, there has been a significant placebo response in atopic dermatitis (25% for a 50% improvement in EASI (called EASI-50) in dupilumab studies (3)), unlike in psoriasis trials where the placebo effect is usually less than 5%. On the other hand, all of these patients treated with GR-MD-02 had more severe baseline scores and refractory disease after multiple therapies, so it may be a more challenging group than those typical difficult-to-treat patients who have failed topical steroids only.
So, how does the effect we saw in this small study compare to other drugs in development? Dupilumab, a drug in development with successful phase 3 trials, averaged 64% of patients reaching an EASI-50 at week 16 of therapy (3). Two out of three of our patients reached an EASI-50 (reduction of EASI of 64% and 74%, respectively) at 6 weeks of therapy (3 doses of drug) which is a shorter period of time than with dupilumab. Moreover, as stated above, the baseline EASI score was more severe in our patients than in the phase 3 dupilumab studies and our patients had been on multiple systemic drugs, whereas those in the dupilumab studies had only to fail topical steroids.
Nemolizumab, another phase 3 drug asset, averaged 40% reduction in EASI at 12 weeks; patient 1 and 3 both exceeded this. Our patients 1 and 3 also faired favorably compared to tofacitinib, which has only been used in one small study, and there is not data on lebrikizumab which is currently in development.
Therefore, it appears that the effects on severe atopic dermatitis seen in our small, open label protocol, compares favorably with the other drugs currently in development.
What’s next?
Several arguments suggest that GR-MD-02 has potential as a therapy for severe atopic dermatitis:
- It represents a new and novel mechanism of galectin-3 inhibition in the disease
- We have seen clinically relevant, preliminary results of GR-MD-02 in three refractory patients with severe atopic dermatitis.
- There is a lack of effective therapy for these patients currently on the market
- GR-MD-02 has apparently similar magnitude of clinical effect levels compared to drugs in development
- GR-MD-02 has a very strong safety profile, with nearly 3,000 doses of GR-MD-02 delivered in multiple completed and ongoing clinical trials with no severe adverse effects attributed to the drug
- While intravenous administration is a potential drawback, it is encouraging that patient 1 asked to continue on therapy for an additional 6 months.
Phase 2, randomized, placebo-controlled trials with various doses and regimens of administration will be necessary to explore this potential. Planning for such a program is ongoing, as the company explores partnership and other options to finance such a program.
Reference List
- Arkwright PD, Motala C, Subramanian H, Spergel J, Schneider LC, Wollenberg A. Management of difficult-to-treat atopic dermatitis. J Allergy Clin Immunol Pract 2013 Mar;1(2):142-151.
- Saegusa J, Hsu DK, Chen HY, Yu L, Fermin A, Fung MA, et al. Galectin-3 is critical for the development of the allergic inflammatory response in a mouse model of atopic dermatitis. Am J Pathol 2009 Mar;174(3):922-931.
- Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N Engl J Med 2016 Dec 15;375(24):2335-2348.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including whether GR-MD-02 may be effective in the treatment of severe atopic dermatitis, psoriasis, and other skin disease. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2015, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.