Galectin Inhibitor Therapy Effective in Severe Atopic Dermatitis (Eczema)
Guest Author I welcome as a co-author to this Perspective, Dr. Simon Ritchie, our lead investigator in skin disease and staff dermatologist, at San Antonio Military Health System, Fort Sam Houston, TX. Dr. Ritchie conducted the clinical study in moderate-to-severe plaque psoriasis and will present the promising results of the effect of our galectin inhibitor […]
The Purpose of the CEO Perspectives Blog
It has now been almost 18 months since I started my CEO Perspectives blog. From the questions and comments I get about my posts, I can tell that many people find it useful and informative. I thought it might be a good time to take a look back at what I’ve covered over the past […]
Galectin-3, a Critical Link in the Cause of Diabetes
Galectin-3 is a protein, made predominantly in immune cells in the body, which binds to specific sugar molecules attached to many other types of proteins (called glycoproteins). By binding to glycoproteins, galectin-3 has many effects, such as modulating the interaction of cells and receptors, the immune response, the behavior of cancer cells, inflammation, and scarring […]
Results from the NASH-FX Study Underscore the Importance of Completing the NASH-CX Clinical Trial for Patients with NASH Cirrhosis
On September 27, 2016, we announced the results of our NASH-FX Phase 2a exploratory clinical study on the use of GR-MD-02 in nonalcoholic steatohepatitis (NASH) patients with advanced fibrosis. While the study did not meet its primary and secondary endpoints, there were aspects of this study that underscore the importance of completing our ongoing NASH-CX Phase 2b […]
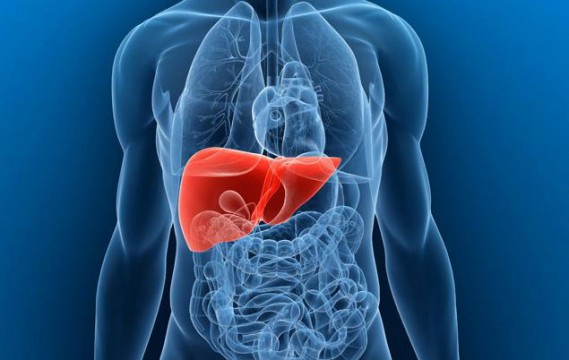
Fatty Liver is an Enormous Global Problem
I often write about fatty liver disease, a problem that is gaining mainstream recognition as published earlier this year in Newsweek. It is easy to assume that this is a problem just in the U.S., but data are coming in that highlight it as a global problem, as published in a recent article (1) and […]
Can Drinking Coffee Cure NASH or Liver Fibrosis?
There was a big splash in the news recently about a study showing coffee drinking prevented cirrhosis. This seems to have struck a chord with people, and I’ve been getting a lot of questions about the study. The short answer is, “Yes, drinking coffee does seem to reduce your chances of developing cirrhosis.” However, it’s […]
Love Your Liver: A Prescription for Liver Health
Galectin Therapeutics is focused on a treatment for advanced fatty liver disease, or NASH (non-alcoholic steatohepatitis), which includes those patients with advanced scarring, or who are at the end stage of scarring called cirrhosis. I am often asked how one can maintain a healthy liver and avoid the complications that can happen with chronic fatty […]
Photographs of Improvement in Moderate-To-Severe Plaque Psoriasis
We recently announced positive interim results from an open-label, Phase 2a clinical trial with GR-MD-02 in patients with moderate-to-severe plaque psoriasis (see press release here). On April 28th, I also published a CEO Perspective (here) on considerations for interpretation of the results of this trial. Today, I want to provide additional commentary on the interim […]
Pulmonary Arterial Hypertension is Associated with Elevated Levels of Galectin-3 and a Potential Therapeutic Target for GR-MD-02
Elevated levels of the Galectin-3 protein are associated with multiple disease processes, in addition to those Galectin Therapeutics is addressing in ongoing clinical trials such as fatty liver disease, psoriasis and cancer. Among those is disease of heart muscle and blood vessels. Galectin Therapeutics is collaborating with a number of academic groups to evaluate cardiovascular […]
How Will We Interpret Results from the GR-MD- 02 Psoriasis Trial?
By Peter G. Traber, M.D. on April 28, 2016 Last September, Galectin Therapeutics began a Phase 2a open-label clinical trial with GR-MD-02, our galectin-3 inhibitor drug, in patients with moderate-to-severe plaque psoriasis. We initiated this trial because a patient in our Phase 1 trial with GR-MD-02 in NASH had a remarkable and sustained remission of […]
Can Thin People Get Fatty Liver Disease? Lean NASH
By Peter G. Traber, M.D. on April 21, 2016 I suppose it’s natural to assume that only overweight people get fatty liver disease (and NASH), but one thing I have learned over my career in medicine is to always challenge such “common sense” assumptions with empirical research. It turns out that, while people who are […]
The Dilemma of Treating NASH
By Peter G. Traber, M.D. on March 24, 2016 You may have seen Newsweek’s recent article on the growing impact of NASH, NASH Is the 21st Century’s Looming Health Threat. It’s a great article that vividly demonstrates the heartbreak of a NASH diagnosis and the shame and uncertainty faced by many patients. “I’m in this […]
The Patent Portfolio Strategy Behind GR-MD-02
By Peter G. Traber, M.D. on March 17, 2016 We often talk about how Galectin Therapeutics’ proprietary compound, GR-MD-02, is protected by a strong intellectual property portfolio. I thought it might be useful to drill a little more deeply into that statement. Our patent portfolio is an important asset for Galectin Therapeutics, and it took […]
Portal hypertension and why it’s important
By Peter G. Traber, M.D. on February 22, 2016 Everyone has had the experience of having their blood pressure taken and knows the importance of a normal reading for good health. But most people have never heard of liver blood pressure. In fact, high liver blood pressure, called portal hypertension, is the primary reason for […]
Developing Tests to Assess Liver Function in NASH and Cirrhosis
By Peter G. Traber, M.D. on February 11, 2016 I wrote recently about the need for non-invasive tests to replace liver biopsies in the evaluation of liver fibrosis due to various diseases, including NASH, and I also spent time exploring some of the non-invasive serum and imaging tests that are in development. I started this […]
Fatty liver disease: Motivation to lose weight
By Peter G. Traber, M.D. on February 3, 2016 Are you one of the nearly 40% of Americans classified as obese or are you overweight and inexorably headed towards obesity? Has your physician ever suggested you lose weight or have you made a New Year’s resolution to go on a diet? Do you need any […]
A Brief Overview of Non-Invasive Testing in Liver Fibrosis
By Peter G. Traber, M.D. on January 28, 2016 I wrote recently about why we rely on liver biopsies for the diagnosis of NASH and liver fibrosis and the need for alternative, non-invasive tests that enable us to diagnose and track the progression the disease. Today I’d like to explore the different non-invasive tests being developed. […]
Why Non-Invasive Testing is Needed in Liver Fibrosis
By Peter G. Traber, M.D. on January 20, 2016 Several of my past CEO Perspectives have touched on the need for a non-invasive test in diagnosing and following treatment of fatty liver disease, non-alcoholic steatohepatitis (NASH), and cirrhosis. Currently, the only broadly accepted way to assess a patient’s condition is via a liver biopsy, an invasive […]
2015 – 2016, Progress and Possibilities
By Galectin Therapeutics. on January 7, 2016 The many accomplishments at Galectin Therapeutics during 2015 ranged from incremental progress touching every aspect of our business to significant advancements with GR-MD-02. Importantly, this progress forms the basis for numerous milestones expected in the coming years including clinical progress, intellectual property fortification, further engagement with the investment community […]
Are You Eligible for the Galectin Therapeutics’ NASH Cirrhosis Trial?
By Peter G. Traber, M.D. on December 7, 2015 In June 2015 Galectin Therapeutics initiated a clinical trial to determine if our investigational drug GR-MD-02 can successfully treat patients with severe scarring of the liver (cirrhosis) due to fatty liver disease (a.k.a. nonalcoholic steatohepatitis, or NASH). The goal of the study (the NASH-CX trial) is […]
Two Clinical Trials Using GR-MD-02 Galectin-3 Inhibitor in Combination with Cancer Immunotherapy Drugs
By Peter G. Traber, M.D. on November 12, 2015 The galectin-3 protein appears to play an important role in the progression of many cancers, especially with respect to the ability of cancer cells to hide from the body’s immune response. With combination immunotherapies becoming increasingly important in the treatment of cancer, inhibiting galectin-3 could provide […]
Cancer Immunotherapy Reaches an Inflection Point
By Peter G. Traber, M.D. on November 5, 2015 To me, the recent FDA approval of the use of Opdivo® (nivolumab) in combination with Yervoy® (ipilimumab) for the treatment of patients with metastatic melanoma represents an inflection point in cancer immunotherapy. The announcement marks the first FDA approval of a regimen combining two immuno-oncology agents […]
The Role of Galectins in Cancer
By Peter G. Traber, M.D. on October 29, 2015 Galectin proteins, particularly galectin-1 and galectin-3, are present in increased amounts in cancers of many types. In fact, expression of galectin-3 appears to be higher in the vast majority of solid tumors including skin, lung, breast, pancreas, ovarian, colon, thyroid and head and neck, among others. […]
Why Fibrosis is a Hot Area for Pharmaceutical Research
By Peter G. Traber, M.D. on October 21, 2015 Bristol-Myers Squibb ($BMS) recently paid $150 million for the exclusive right to acquire Promedior, a clinical-stage biotechnology company in the midst of a Phase 2 trial on the treatment of idiopathic pulmonary fibrosis (IPF) and myelofibrosis (MF). If clinical trials are successful, the deal could be […]
Chemistry, Manufacturing and Controls (CMC) for GR-MD-02
By Peter G. Traber, M.D. on October 15, 2015 When thinking about the development of a drug, one naturally tends to think of performing animal and human studies to demonstrate the safety and effectiveness of the drug in the intended disease indication. However, a drug development program not only includes these safety and efficacy studies, […]
Lessons Learned from the Failure of Raptors’ Phase 2b NASH Trial
Written by Peter G. Traber, M.D. on October 8, 2015 On September 14, 2015, Raptor Pharmaceutical Corp. (NASDAQ: RPTP) announced top-line results of its Phase 2b trial evaluating RP103 in children with biopsy-confirmed nonalcoholic steatohepatitis (NASH). As reported here, the study failed to meet its primary endpoint of a two-point decrease in a fatty-liver-disease scoring […]
Exploratory Indication in Psoriasis
Written by Peter G. Traber, M.D. on September 24, 2015 Serendipity, or fortunate happenstance, has always been an important part of the search for medicines. As pointed out by Thomas A. Ban (1), serendipity in drug discovery is more than just luck, but rather is best described by Pasteur’s famous quote that “Chance favors the […]
Second Phase 2 clinical trial initiated for fatty liver disease with advanced fibrosis
Written by Peter G. Traber, M.D. on September 16, 2015 As a company, we are focused on developing a therapy for patients with non-alcoholic steatohepatitis (NASH), or fatty liver disease, with advanced fibrosis (scarring) of the liver. Our drug GR-MD-02 has been shown to prevent and reverse fibrosis in preclinical animal models and there was […]
Lead product candidate GR-MD-02 shows no unfavorable drug-drug interactions
Written by Peter G. Traber, M.D. on September 9, 2015 An important question in drug development, and the subject of U.S. Food and Drug Administration (FDA) regulations, is whether a drug candidate interacts with other medications the target patient population may also be taking. This is a critical issue, because many patients with chronic diseases […]
Clinical Trial to Establish Efficacy of GR-MD-02 in NASH Cirrhosis
Written by Peter G. Traber, M.D. on September 2, 2015 In late June 2015, Galectin Therapeutics initiated a Phase 2 clinical trial (the NASH-CX trial) to evaluate the ability of its anti-galectin drug GR-MD-02 to treat patients with cirrhosis of the liver due to fatty liver disease (non-alcoholic steatohepatitis, or NASH). Cirrhosis is advanced-stage scarring, […]
Successful Phase 1 Clinical Trial Supports Phase 2 Clinical Development Program
Written by Peter G. Traber, M.D. on August 24, 2015 Galectin Therapeutics completed and reported on a successful Phase 1 clinical trial in NASH patients with advanced fibrosis. In a previous “CEO Perspective”, I described the general outline of our Clinical development program. We are using the galectin-3 inhibitor GR-MD-02 in a development program for […]
Clinical Development Program in Liver Fibrosis
Written by Peter G. Traber, M.D. on August 17, 2015 Galectin Therapeutics is developing a drug called GR-MD-02 which binds to and inhibits the galectin-3 protein which is important in the promotion of multiple types of disease including inflammation, fibrosis, and cancer. We have focused the use of this drug in a development program for […]
The Potential for Treatment of Liver Fibrosis With Galectin’s Drug Candidate GR-MD-02
Written by Peter G. Traber, M.D. on July 31, 2015 Liver fibrosis is scar formation in the liver which, when it reaches a large amount of scar tissue (called cirrhosis), results in liver failure and complications leading to death. The only available treatment for liver cirrhosis is a transplant, which is not only expensive, but […]
Welcome to CEO Perspectives
Written by Peter G. Traber, M.D. on July 31, 2015 During the normal course of conducting business in a public company like Galectin Therapeutics, as the CEO and Chief Medical Officer of the company, I have conversations and interactions with many different firms and individuals on a daily basis. I am frequently discussing and fielding […]