We recently announced positive interim results from an open-label, Phase 2a clinical trial with GR-MD-02 in patients with moderate-to-severe plaque psoriasis (see press release here). On April 28th, I also published a CEO Perspective (here) on considerations for interpretation of the results of this trial. Today, I want to provide additional commentary on the interim results, including photographs demonstrating disease improvement.
Patients treated with GR-MD-02 experienced significant clinical improvement
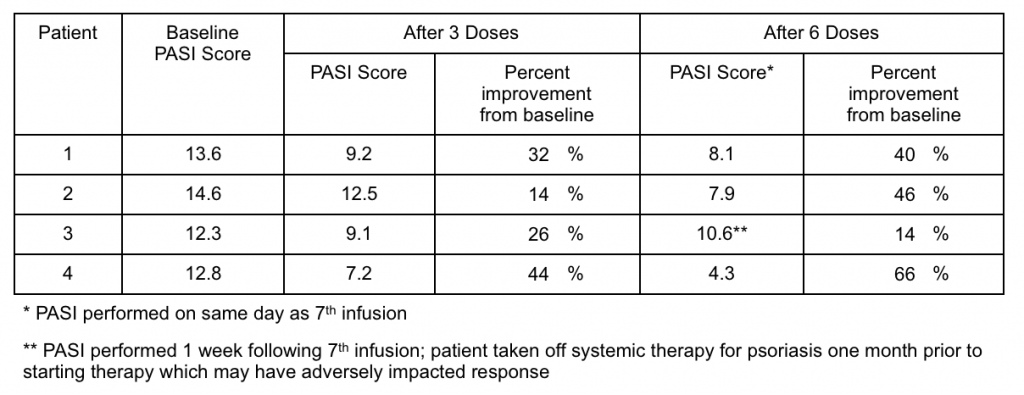
As described in the press release, each of the four patients who received six doses of GR-MD-02 over 12 weeks of therapy reported improvement in their psoriasis symptoms. Moreover, three patients had significant, and one had modest, improvement in the Psoriasis Area Severity Index (PASI), which is an objective and standardized means of evaluating disease response (see table below).
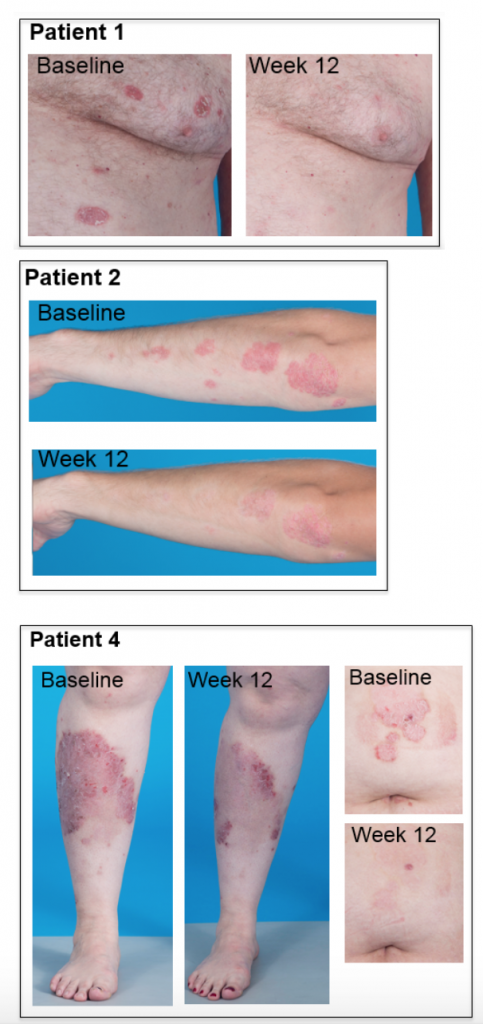
In addition to these quantitative results, photographs provide vivid evidence of response to therapy (below). Each patient had over 70 pictures taken at each photography session, so this represents only a small sample and not a full representation of their whole body disease. These photographs provide evidence of disease improvement in areas of significant improvement.
Patient 1 had complete clearing of multiple chest lesions. Patient 2 had near complete resolution of arm and elbow lesions. Patient 4, the one with the greatest reduction in PASI score, had nearly complete resolution of an abdominal lesion and remarkable improvement of very severe leg lesions. Most of the disease on Patient 4’s leg is actually completely resolved with only some skin hyperpigmentation, which generally takes months to slowly fade.
What does this mean for our drug GR-MD-02?
It is uncommon for patients with moderate-to-severe plaque psoriasis to spontaneously improve without treatment, so the improvements we have seen in this trial are due to GR-MD-02. Furthermore, these improvements are clinically significant for these patients, as all of them suffered from moderate-to-severe psoriasis for years (between 6 and 40 years) prior to entering the trial. Therefore, it is our belief that GR-MD-02 had a positive effect on psoriasis in these patients.
We believe this is the first definitive clinical effect of GR-MD-02 to be shown in patients. While all diseases are not the same in terms of etiology, these results support our optimism for GR-MD-02 to affect other disease processes that the company is investigating.
How do our results with GR-MD-02 compare with other available psoriasis therapies?
Beyond the fact that GR-MD-02 has a biological effect on psoriasis in patients, we need to consider the possibility that GR-MD-02 could be additive to the medical armamentarium for treating psoriasis. There are multiple commercial and development-stage drugs for the treatment of moderate-to-severe plaque psoriasis, including biological agents that would constitute the direct competition for GR-MD-02.
There is an established FDA regulatory pathway for drug approval in moderate-to-severe plaque psoriasis that all the recent marketed biological drugs have followed. PASI is universally used in registration clinical trials (also referred to as phase 3 or pivotal clinical trials) as an objective measurement of drug response. All registration clinical trials assess the number of patients who achieve a 75% improvement in PASI (called PASI-75) at various times following initiation of therapy. For currently approved drugs, the percentage of patients who achieve PASI-75 at between 12 and 24 weeks of therapy ranges from 54% to as high as 90% in some studies.
At the time of this interim analysis of our trial, none of the four patients had reached a PASI-75, which is why we have taken the next steps described below.
What are the next steps?
We have extended therapy in the study with the current dose of GR-MD-02 for a full 24 weeks, or 13 infusions, to determine whether improvement at this dose continues and reaches PASI-75. Extension beyond 24 weeks of therapy to reach PASI-75 would not be competitive from an efficacy standpoint among the currently marketed biological agents for moderate-to-severe plaque psoriasis. However, in general, approval of drugs is a balance between efficacy and safety and most of the drugs on the market have serious side effect profiles. We are now attempting to enroll the full cohort of 10 patients in this trial, with all receiving 24 weeks of therapy. We will continue to report interim data from this trial.
This interim analysis of four patients has given us great encouragement on the biological effect of GR-MD-02 in a human disease and we look forward to reporting results later this year on this trial and our trial with GR-MD-02 in patients with NASH and advanced (stage 3) fibrosis (the NASH-FX trial).
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including whether GR-MD-02 may be effective in the treatment of psoriasis. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2015, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.