By Peter G. Traber, M.D. on April 28, 2016
Last September, Galectin Therapeutics began a Phase 2a open-label clinical trial with GR-MD-02, our galectin-3 inhibitor drug, in patients with moderate-to-severe plaque psoriasis. We initiated this trial because a patient in our Phase 1 trial with GR-MD-02 in NASH had a remarkable and sustained remission of her psoriasis following treatment, as described in a previous CEO Perspective (here). Since then, I have been asked on numerous occasions how we will interpret the results of this trial.
What are the psoriasis trial endpoints?
Multiple drugs have been approved for the treatment of psoriasis, so there is an established FDA regulatory pathway to approval. It is well accepted that assessment of drug efficacy in psoriasis clinical trials is best performed using the Psoriasis Area and Severity Index, or PASI. PASI is used both to measure the severity of psoriasis as well as to measure the effect of treatment. While this index score is not generally used in the clinical practices of dermatology, it is universally used in clinical trials as an objective measurement of drug response.
PASI is an objective set of measurements that are conducted by a physician to assess the area of skin involvement and the severity of skin lesions in patients with psoriasis. The figure below, taken from a review article written by experts in the field, outlines the approach to the measurement (1).
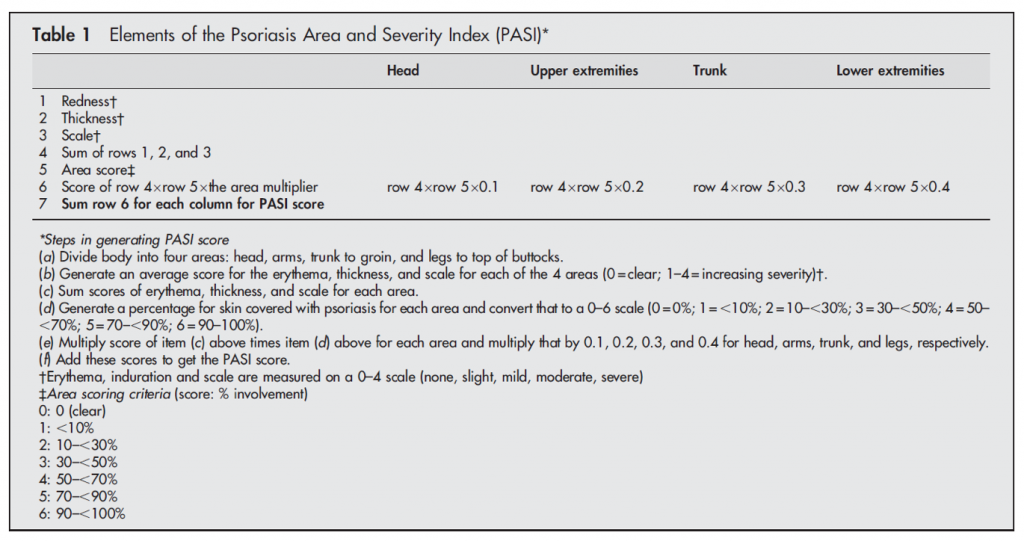
Therefore, the primary endpoint in our psoriasis trial will focus on improvement in PASI following treatment with GR-MD-02. We will evaluate the number of patients who have various percentage improvements in PASI. We will also record the durability of response to treatment for up to one year, should there be a beneficial effect. Finally, we are monitoring the incidence of adverse events and vital sign and laboratory abnormalities, if any, during study treatment.
What constitutes a meaningful effect?
Moderate psoriasis is generally defined as a PASI of ≥10. In our trial, as is typical for clinical trials in moderate-to-severe psoriasis, patients who enroll in the trial are required to have at least 10% of their body area affected by psoriasis and a PASI of ≥12, parameters which were agreed to with the FDA. Moreover, the patients will have the diagnosis of psoriasis confirmed by a skin biopsy. Therefore, patients in the trial will have significant disease and are similar to those who have been enrolled in the trials of approved drugs for psoriasis.
Once an individual has established psoriasis, it generally does not spontaneously dissipate or improve. As published in the medical literature, it has been suggested that clinically significant improvements in psoriasis are clearly obtained when PASI is lowered by 50% of baseline values, i.e., a PASI 50 response (2). However, the accepted clinical trial endpoint for drug registration is PASI 75, meaning a 75% reduction in psoriasis versus baseline. However, since this is an exploratory trial in which we do not have any information on optimal dosing, we will be looking for any objective response.
What will this trial tell us and what might be the next steps?
First and most important, our exploratory psoriasis clinical trial will either confirm the effect on psoriasis seen in the one Phase 1 patient, or show that her remission was unrelated to our drug. Should a significant proportion of patients in the trial respond to GR-MD-02 with an improvement in their psoriasis, such a finding would represent the first human disease response to GR-MD-02. That would be an important finding with potential implications for activity in our main therapeutic program for NASH, a disease where there is a higher incidence of psoriasis.
Second, this trial may indicate whether GR-MD-02 is a viable candidate to advance as a therapy for psoriasis. There are a number of biologic agents that have been approved in recent years for moderate-to-severe psoriasis, and GR-MD-02 would need to be competitive with those drugs. In my view, based on the efficacy of approved drugs, GR-MD-02 would need to demonstrate a PASI 75 in at least 50% of treated patients to consider full clinical development. If it does have such a degree of efficacy, it might be considered as a potential drug therapy on the basis of its lower cost to manufacture than existing drugs, a potentially better safety profile or the duration of effect after stopping treatment.
While each of these potential benefits would need to be studied in detail, the first step is to determine whether the drug has an effect on the disease, which is the primary goal of this study.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including whether GR-MD-02 may be effective in the treatment of psoriasis. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2015, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Reference List
1. Feldman SR, Krueger GG. Psoriasis assessment tools in clinical trials. Ann Rheum Dis 2005 Mar;64 Suppl 2:ii65-ii68.
2. Carlin CS, Feldman SR, Krueger JG, Menter A, Krueger GG. A 50% reduction in the Psoriasis Area and Severity Index (PASI 50) is a clinically significant endpoint in the assessment of psoriasis. J Am Acad Dermatol 2004 Jun;50(6):859-866.