The post Results from the NASH-FX Study Underscore the Importance of Completing the NASH-CX Clinical Trial for Patients with NASH Cirrhosis appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The NASH-FX study was designed as a pilot study at a single site, involving only four months of treatment with GR-MD-02. This pilot study evolved from the results of a Phase 1 study in NASH patients with advanced fibrosis that had suggested FibroScan® measurements improved in three patients with just four doses of the study drug. The full report of this Phase 1 study will be published in the next few weeks in the peer-reviewed scientific journal, Alimentary Pharmacology and Therapeutics.
While most experts feel that liver fibrosis trials should have treatment phases for at least a year in duration, the results from the earlier Phase 1 NASH study provided a rationale for studying a larger group of patients with shorter therapy and exploring non-invasive technologies for assessing liver disease and fibrosis with a goal of using these technologies in later trials.
I want to give you a few details on the design of the NASH-FX study. The primary endpoint of NASH-FX was the LiverMultiScan (LMS), an approved magnetic resonance imaging test developed by Perspectum Diagnostics to assist in the diagnosis of liver disease. In NASH, the LMS is reported to measure the amount of fibrosis and inflammation. The LMS has low variability between scans in the same individual, and we used this low variability to calculate the number of patients we would need for the study to show a statistically significance difference between treatment groups with a power of 80%. In other words, the study was designed to have an 80% chance of showing a statistically significant difference in a 30 patient study, with 15 placebo and 15 GR-MD-02 patients. The power of the actual study, calculated after completion, was almost exactly an 80% chance to show an approximately 10% difference in LMS between placebo and treated groups. Therefore, the study design was adequate for the primary endpoint.
In contrast to LMS, the study was not powered for the secondary endpoints of liver stiffness, FibroScan and magnetic resonance elastography (MRE). The study would have required between 3 to 5 times as many patients to have an adequate power to show a difference with these tests. This is because the variability of these tests for repeated measurements is considerably greater than LMS.
However, we did not know before we conducted the study, nor did anyone else know, whether LMS correlated with the liver stiffness measurements of FibroScan and MRE. The NASH-FX study showed that there was poor correlation. Therefore, one cannot conclude that because there was no difference in LMS, that there would not be a difference in stiffness measurements, which have been shown in liver biopsy studies to correlate with fibrosis.
Although there was no apparent improvement in the three non-invasive tests for assessment of liver fibrosis in the four-month NASH-FX study, Dr. Stephen Harrison, a leading investigator in NASH and liver disease and the principal investigator of the NASH-FX study has pointed out that the inhibition of galectin-3 with GR-MD-02 remains promising for the treatment of NASH fibrosis. Dr. Harrison was especially encouraged that GR-MD-02 has demonstrated an improved clinical effect in moderate-to-severe psoriasis, suggesting the compound has activity in a human disease that can occur in association with NASH.
It remains critical that we complete the longer therapy NASH-CX clinical trial that has a much larger group of patients with NASH cirrhosis.
The NASH-CX trial is a one-year of treatment, multi-center trial in patients with NASH cirrhosis that is being conducted at 36 outstanding liver centers in the United States. The endpoints of the NASH-CX trial include invasive tests that are well-validated measures of liver disease severity. The primary endpoint is the change from baseline in the hepatic venous pressure gradient (HVPG), which measures the blood pressure in the liver and is well correlated with the clinical outcomes of patients.
Liver biopsy is an important secondary endpoint in the NASH-CX trial, which evaluates the stage of liver fibrosis and the amount of collagen, the primary component of fibrotic tissue. Finally, there are also non-invasive tests as secondary endpoints, including FibroScan and the 13C-methacetin breath test, which measures the metabolic function of the liver. These are important to correlate with the invasive tests because they may be useful in future trials and in management of patients.
I am pleased to report additional information on the status of this most important clinical trial as of October 10, 2016:
- The NASH-CX trial completed enrollment one month early with 162 total patients, exceeding the target of 156. This keeps us well on track for reporting of top-line results in December of 2017.
- The 162 patients were enrolled at 36 sites in the United States following the screening of 290 patients to obtain a population with well-compensated NASH cirrhosis (Child-Pugh-Turcotte Class A) with elevated portal pressure (HVPG ≥ 6 mmHg).
- In determining the number of patients to meet statistical requirements, we planned for the possibility that as many as 25% of the patients may drop out of the study during the treatment phase. However, we are pleased that only five patients of the 162 enrolled have dropped out of the study thus far. This low attrition rate highlights the importance, urgency, and need for patients suffering from NASH-cirrhosis to find an effective medical treatment.
- The low drop out trend also suggests that we will have a robust number of patients completing treatment for evaluation at the end of the trial. The trial was designed to have an 80% chance of demonstrating a 2 mmHg reduction in HVPG (i.e. 80% power) with 117 patients evaluated. Any number of patients above 117 will simply enhance the power of the study.
- At this point, 4 patients have completed the entire protocol and 70 patients have already completed six months of dosing.
- A total of 2,000 drug infusions (including placebo) have been given in this trial, representing 48% of the total number of infusions in the entire study. So we are quite pleased that this study is well along in its development.
The safety and tolerance of GR-MD-02 in all of the trials is most encouraging and supports our commitment to pursue the lead indication of NASH cirrhosis. In the NASH-FX study, GR-MD-02 was found to be safe and well-tolerated among the patient population with no serious adverse events related to the study medication. Over all of the clinical trials, including the patients in the NASH-CX trial, more than 1600 doses of GR-MD-02 have been administered without serious adverse effects related to the drug. This highlights the superior safety profile of the therapy in a patient population with advanced-stage disease, which is buttressed by the biological activity demonstrated in patients with moderate to severe plaque psoriasis.
Dr. Harrison, one of the two co-lead investigators in the NASH-CX trial, stated his belief that the inhibition of galectin-3 with GR-MD-02 remains a promising treatment for NASH fibrosis and that it is important to complete the NASH-CX trial.
Dr. Naga Chalasani, the other co-lead principal investigator of the NASH-CX trial, provided his assessment, stating:
“The results from the NASH-FX trial do not diminish the significance of the NASH-CX trial. Along with the safety and tolerability profile observed in the NASH-FX trial, the different patient population, much larger enrollment, rigorous study design and longer duration of therapy offer compelling rationale to complete the NASH-CX trial.”
As a company, Galectin Therapeutics’ attention has always been focused on completing the NASH-CX clinical trial and reporting results in a timely fashion.
With an outstanding safety profile, inhibition of galectin-3 with GR-MD-02 remains a potential treatment of NASH cirrhosis. Additionally, the longer therapy for one year, and endpoints that may serve as a surrogate for outcomes for registration trials in this patient population, provides us encouragement about our continuation of NASH-CX clinical trial.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including whether GR-MD-02 may be effective in the treatment of NASH. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2015, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Make a Comment or Ask a Question
[contact-form-7]The post Results from the NASH-FX Study Underscore the Importance of Completing the NASH-CX Clinical Trial for Patients with NASH Cirrhosis appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Can Thin People Get Fatty Liver Disease? Lean NASH appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>I suppose it’s natural to assume that only overweight people get fatty liver disease (and NASH), but one thing I have learned over my career in medicine is to always challenge such “common sense” assumptions with empirical research. It turns out that, while people who are overweight or obese are indeed at greater risk, thin people can also develop fatty liver disease, NASH and cirrhosis. This has come to be known as “lean NASH.”
The commonly accepted definition of obesity is a body mass index (BMI) of 30 or more. However, overall BMI is less important in determining risk for fatty liver disease than where the fat is located in the body. Visceral fat, when the fat is nestled in and around the organs of the belly, is more strongly linked to fatty liver disease and other metabolic disorders than fat in arms, legs, and other parts of the body. A person can easily have a BMI well below 30 and still have considerable visceral fat. Someone who is “metabolically obese, normal weight” will have many of the hallmarks of obesity, such as insulin resistance (requirement of higher insulin levels to control blood sugar), metabolic syndrome (high blood sugar, elevated fats and cholesterol in blood, high blood pressure, and excess body fat around the waist) and NASH.
A study published in 2012 showed that overall prevalence of fatty liver disease among obese individuals was 28 percent, while fatty liver disease was identifiable in 7 percent of the lean individuals tested. (Zobair M. Younossi, 2012) Yes, obese people had a greater incidence of fatty liver disease, but lean people still showed a surprisingly high prevalence of the disease.
The other surprising finding from this study was that lean NASH patients here in the U.S. tend to be Hispanic. It’s unclear whether it is culture, diet, genetics, or some completely different mechanism at work that makes those of Hispanic ancestry more likely to develop lean NASH. But, given the growth of the Hispanic population here in the U.S., lean NASH is likely to emerge in the coming decades as an important cause of chronic liver disease.
Ethnicity does seem to be one of the major determinants of lean NASH, which was first described by physicians in Asia. While metabolic syndrome has long been a problem in developed countries, it is an increasing problem in developing countries as well, even though the rate of obesity remains comparatively low. The prevalence of fatty liver disease among normal-weight individuals was recently reported at 12% in Greece, 20% in India and 15% in China.
One analysis from 2013 suggests that lean NASH, as seen in Asia, is a distinct phenotype of NASH (Kausik Das, 2013). Asians, this study notes, show a propensity to develop metabolic syndrome at a lower BMI. One possible reason is that early malnutrition, either in utero or in early childhood, primes the body to store visceral fat more aggressively. The relative abundance of food in Asia today over the scarcities common only a few decades ago means that adults in China, India and other Asian countries are increasingly at risk of developing lean NASH.
I’ve called fatty liver disease a “hidden epidemic,” and lean NASH is even more so. It is easy to identify someone with a high body weight as being at risk for fatty liver disease, but less so for someone with lean NASH. We need a much better understanding of what causes lean NASH and how its presentation and biomarkers are distinct from the fatty liver disease and NASH seen in overweight patients.
Works Cited
- Kausik Das, A. C. (2013). Lean NASH: distinctiveness and clinical implication. Hepatol Int , 7(Supplement 2), S806 – S813.
- Zobair M. Younossi, M. M. (2012, November). Nonalcoholic Fatty Liver Disease in Lean Individuals in the United States. Medicine, 91(6), 319-227.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2015, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Make a Comment or Ask a Question
[contact-form-7]The post Can Thin People Get Fatty Liver Disease? Lean NASH appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Portal hypertension and why it’s important appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>Everyone has had the experience of having their blood pressure taken and knows the importance of a normal reading for good health. But most people have never heard of liver blood pressure. In fact, high liver blood pressure, called portal hypertension, is the primary reason for complications and death in patients with advanced chronic liver disease, called cirrhosis.
The liver has a dual blood supply, which is different than most parts of the body. For example the kidney, intestines, muscles and many other tissues have a single blood supply that comes directly through arteries via the heart. The liver has two blood supplies — one directly from an artery (similar to other tissues), and a second blood flow from the portal vein. The greater proportion of blood comes from the portal vein, which drains the blood from the abdominal organs (stomach, small and large intestine, pancreas, and spleen).
Portal blood brings nutrients and hormones from the gastrointestinal tract which are important for liver function and homeostasis of many bodily functions. After arterial and portal blood mixes and flows through the liver, it returns to the heart via the main vein in the body.
Liver disease has a profound effect on this blood flow. All chronic liver disease leads to scar formation, or fibrosis. Whether the disease is due to a virus, alcohol, or fat, this scar tissue accumulates in the liver, with the most advanced stage called cirrhosis. The fibrotic tissue of cirrhosis distorts the liver architecture, impeding liver blood flow and, as a result, the blood pressure in the portal vein increases — this is called portal hypertension.
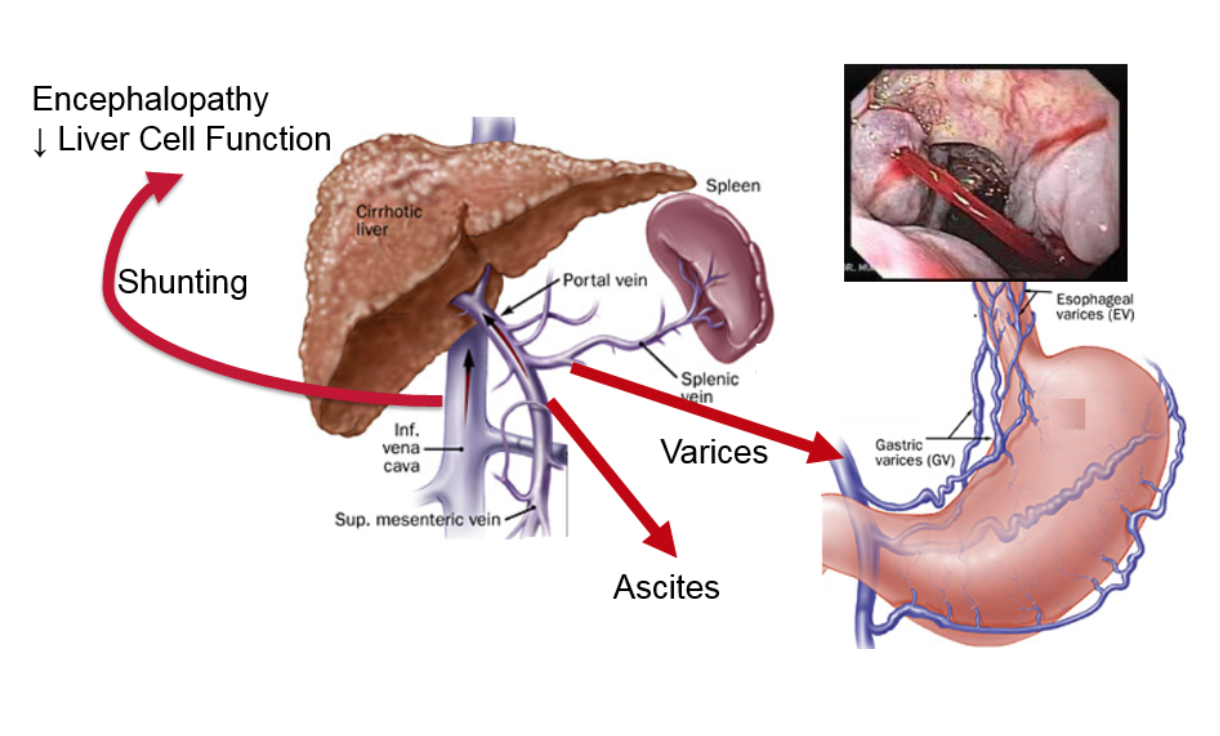
The figure shows what happens in portal hypertension. The increased pressure causes a certain type of vein dilations (similar to varicose veins or hemorrhoids), the most important of which are called esophageal varices. These dilated veins in the esophagus can burst and cause catastrophic bleeding. Another result of increased pressure is the build-up of fluid in the abdomen outside of the organs, called ascites, which is uncomfortable and can become infected. The increased pressure also results in additional vessels opening up, allowing blood to bypass the liver and enter directly into systemic circulation. Toxins that are normally removed by the liver gain direct access to the rest of the body and the brain, causing mental problems such as lethargy, confusion, and, in the worst cases, coma.
Portal hypertension does not occur with early stages of fibrosis but only when cirrhosis develops. In cirrhotic patients, the level of the portal pressure is directly related to the rate of cirrhotic complications and mortality. Additionally, if the portal pressure is decreased, the outcomes of patients are improved. This makes the portal pressure a potential surrogate for outcomes in clinical trials. If a drug therapy reduces portal pressure, it portends a better prognosis for the patient.
Measurement of portal pressure is not as simple as measuring systemic blood pressure. In fact, there is no direct way to measure portal pressure in clinical medicine. However, there is a minimally invasive radiology procedure that gives a very good estimate of portal pressure, called hepatic venous pressure gradient (HVPG). Rather than describe this technique, watch a short video of the procedure.
HVPG is the primary endpoint in our NASH-CX clinical trial, in which patients with cirrhosis are being treated with our galectin-3 inhibitor GR-MD-02 (see details of trial here). The goal of this trial is to reduce fibrosis in the liver, which will reduce the resistance to blood flow through the liver and, as a result, will reduce portal pressure and improve patient outcomes. It is important that we are also doing other tests for liver fibrosis and function, including liver biopsy (more here), FibroScan (more here), and the methacetin breath test (more here).
Measurement of HVPG to assess portal hypertension is a vital tool in evaluating potential therapies for liver cirrhosis. HVPG has been shown to be directly related to patient outcomes, making it a potential surrogate for drug registration trials.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Make a Comment or Ask a Question
[contact-form-7]The post Portal hypertension and why it’s important appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Why Non-Invasive Testing is Needed in Liver Fibrosis appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>Several of my past CEO Perspectives have touched on the need for a non-invasive test in diagnosing and following treatment of fatty liver disease, non-alcoholic steatohepatitis (NASH), and cirrhosis. Currently, the only broadly accepted way to assess a patient’s condition is via a liver biopsy, an invasive method that is fraught with both potential side effects and inaccuracies. It would be a major benefit for physicians and patients if there was a simple, non-invasive test that would enable us to diagnose and track the progression of disease without resorting to biopsy.
There are a number of reasons why we lack a good non-invasive test for NASH and liver fibrosis. Some of them relate to the nature of the liver itself, others to the nature of the disease. In my next few blog posts, I’d like to explore these issues and review some of the more promising approaches for developing an effective non-invasive test for NASH and liver fibrosis.
The liver is different than other organs
There are characteristics of the liver that make it very different than other organs. For example, while the liver does many important things for the body, it’s hard to measure its functions. You can easily assess the function of the heart by measuring the heart rate and blood pressure, or the lungs by measuring the breathing rate, the oxygenation of the blood and asking the patient whether they are having shortness of breath. For the liver, it’s not easy to determine how it’s functioning from either physical exams or talking to the patient.
We also only have one liver, and it’s so important that it has a tremendous reserve capacity. You can knock out a high percentage of the liver and it still functions effectively to keep people alive. It’s a very resilient organ, and, for that reason, diseases can affect the liver for a long time before the liver function starts to fail. That’s one of the reasons why NASH patients can have inflammation in their liver and progressing fibrosis for decades with no or minimal symptoms and no adverse effects until late in the disease progress. Seemingly suddenly, they have cirrhosis.
These two characteristics – the lack of visibility to function and the large reserve capacity – make it difficult to assess liver function in chronic diseases. For instance, one of the functions of the liver is to remove hemoglobin pigments, which are formed into bilirubin and excreted by the liver into the gut. When this function backs up, it causes jaundice. Acute situations like acute viral hepatitis or a blocked bile duct will cause jaundice almost immediately. With a chronic liver disease like NASH, a patient won’t become jaundiced until very late in the disease. A large proportion of the liver can be compromised, and it can still excrete an adequate amount of bilirubin. The same holds true with the drug and glucose intermediary metabolism performed by the liver.
Why we rely on liver biopsy, and why we need something better
Because it is difficult to assess liver function, biopsy has been the mainstay for liver diagnosis for many years. Liver biopsy is often very important for assessing the cause of liver disease (etiology) and the extent of the disease (i.e., the degree of fibrosis). In fact, you cannot definitively determine whether somebody has NASH without doing a liver biopsy.
However, liver biopsies are invasive and are not without risk. The liver resides under the right diaphragm, and part of it is above the ribs and part of it below. In a liver biopsy, a needle is inserted between the ribs and then into the liver. This is a large needle, around twice the diameter of the needles used to draw blood. When the needle is pulled out, a core of liver tissue remains inside of the needle, and that is what is used for the evaluation. While a patient’s side and ribs will be numbed for the procedure, there is no way to numb the surface of the liver, so the biopsy procedure can be painful.
Then there are complications associated with performing the biopsy. The worst complication is bleeding. If the liver gets ripped and bleeds into the abdominal cavity, it can be catastrophic. The needle can also hit other things by mistake, such as the gall bladder. If the gall bladder leaks bile out into the abdomen, it could lead to emergent surgery. A liver biopsy is not a pleasant test, and there are potentially bad complications.
Despite our reliance on liver biopsies as the sole tool for definitive diagnosis of liver fibrosis, there are also potential problems in the accuracy of the findings, particular in assessing the extent of fibrosis. First, the biopsy tests only 1/50,000th of the liver, and the damage in liver fibrosis is usually heterogeneously distributed (patchy) throughout the liver. Given the large functional reserve of the liver, is it is difficult to be completely certain that such a small sample is representative of the overall health of the organ. Interpretation of the sample is also somewhat subjective. While many histological determinations are straightforward, determination of the degree of inflammation, bile duct damage and severity and location of fibrosis is left to the judgment of the pathologist.
Liver biopsies are now uncommon, but that may change
Relatively few liver biopsies are currently being performed in the U.S. Given that there is no current treatment for NASH, many doctors and patients decide that undergoing a liver biopsy is an unnecessary risk. A biopsy might confirm a diagnosis of NASH, but it will not change treatment at all.
As treatments do become available, we will need a better way of diagnosing NASH and importantly following its treatment. We have run into this issue in our NASH-CX clinical trial. We perform a liver biopsy of the participants at the beginning of treatment — to confirm a diagnosis of NASH and provide a baseline to compare against after treatment with GR-MD-02 — but it is simply too invasive and too risky to perform biopsies during the course of treatment. We are using other methods to evaluate progress during the studies, followed by a final biopsy at the end of the studies to confirm what was being observed.
In future CEO Perspectives, I will explore some of the non-invasive tests that are being developed.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. These statements include those regarding the hope that Galectin Therapeutic’ s development program for GR-MD-02 will show that it can be both safe and effective when used in combination with other drugs for the treatment of patients with cancer. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Make a Comment or Ask a Question
[contact-form-7]The post Why Non-Invasive Testing is Needed in Liver Fibrosis appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Are You Eligible for the Galectin Therapeutics’ NASH Cirrhosis Trial? appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>In June 2015 Galectin Therapeutics initiated a clinical trial to determine if our investigational drug GR-MD-02 can successfully treat patients with severe scarring of the liver (cirrhosis) due to fatty liver disease (a.k.a. nonalcoholic steatohepatitis, or NASH). The goal of the study (the NASH-CX trial) is to assess whether GR-MD-02 may reduce the fibrous tissue that is clogging the liver in subjects with NASH cirrhosis. A reduction, in turn, is expected to improve liver function and have a positive effect on patient outcomes, such as perhaps delaying or avoiding a liver transplant. Please see my earlier CEO Perspective, “Clinical Trial to Establish Efficacy of GR-MD-02 in NASH Cirrhosis,” where this study is described in more detail.
Can you participate in this clinical trial?
If your cirrhosis is caused by NASH and you have portal hypertension, but you haven’t developed other complications related to your cirrhosis, then you might be eligible to participate in this trial. You must be between 18 and 75 years old and meet various other study eligibility requirements. This study is limited to people with cirrhosis only caused by NASH and not by any other factors, like alcohol or hepatitis.
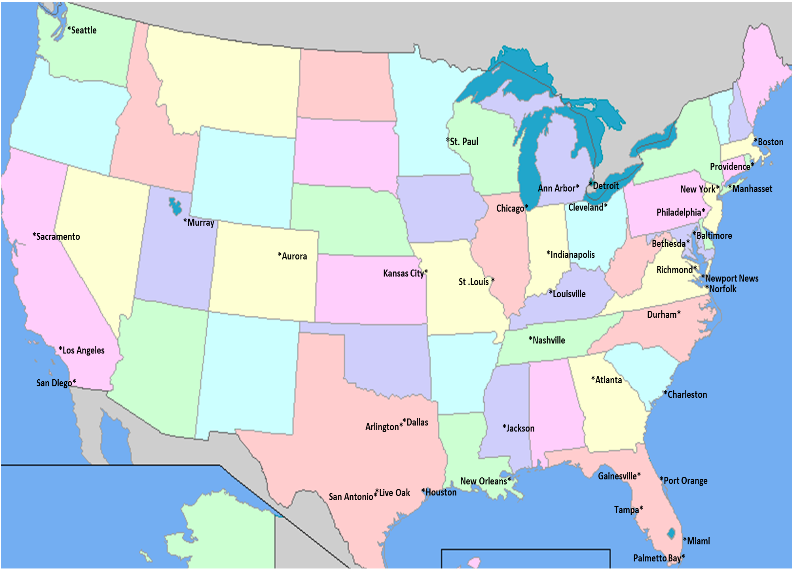
If you qualify, you would be one of 156 participants at about 45 to 60 study sites, or clinics, across the United States. It is important to remember that this is an experimental study comparing GR-MD-02 against a placebo; the trial design is such that you have 2 out of 3 chances of being on active drug and 1 out of 3 chances of being treated with placebo.
The study medication will be administered by an intravenous infusion every two weeks for one year. We expect that each clinic visit will last about an hour and a half. Below you will find a map showing the cities where the study is being conducted to see if you are able to make it to the site every two weeks. If you think you might be eligible for our study, please contact one of the study sites (click here for the list of sites and the appropriate contact information).
More information about this trial can be found at clinicaltrials.gov:
• NASH-CX: https://clinicaltrials.gov/ct2/show/NCT02462967?term=GR-MD-02&rank=3
Should the NASH-CX trial be successful, this will be the first clinical study of an investigational drug candidate to show a reduction of fibrosis in people with cirrhosis and the first hope of a future approved treatment other than a liver transplant.
These “CEO Perspectives” will be a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “could,” “expect” and others. These statements include those regarding the hope that Galectin’s development program for GR-MD-02 will lead to the first therapy for the treatment of fatty liver disease with advanced fibrosis and cirrhosis. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements
Please look for future editions in which multiple aspects of our development programs for unmet medical needs will be addressed.
GT-026 STUDY SITE MAP
Make a Comment or Ask a Question
[contact-form-7]The post Are You Eligible for the Galectin Therapeutics’ NASH Cirrhosis Trial? appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Clinical Trial to Establish Efficacy of GR-MD-02 in NASH Cirrhosis appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>In late June 2015, Galectin Therapeutics initiated a Phase 2 clinical trial (the NASH-CX trial) to evaluate the ability of its anti-galectin drug GR-MD-02 to treat patients with cirrhosis of the liver due to fatty liver disease (non-alcoholic steatohepatitis, or NASH). Cirrhosis is advanced-stage scarring, or fibrosis, in which the liver is “clogged” with fibrous tissue that distorts the organ’s architecture and results in increased resistance to blood flow to the liver and dysfunction of liver cells. The goal of therapy with GR-MD-02 is to reduce fibrosis and, in turn, improve liver function and positively affect patient outcomes.
One of the most important aspects of any clinical trial is the “endpoint” that will be measured as evidence of the drug’s effect. Endpoints include the “primary” endpoint on which the overall success of the trial is based, and “secondary” endpoints that are supportive of the primary endpoint. The strongest endpoints for any clinical trial are those related to patient outcomes which include how a patient feels, functions, or survives (mortality). However, these endpoints in chronic diseases often can only be measured in the end stage of disease, which often occurs many years after the patient contracts the disease. Therefore, many clinical trials are conducted with surrogate endpoints that are linked to patient outcomes. For drugs such as GR-MD-02 that fulfill a significant unmet need, the U.S. Food and Drug administration (FDA) may allow the initial approval of drugs based on achieving a reasonable surrogate endpoint under an accelerated approval pathway.
For NASH cirrhosis, potential surrogate endpoints have been proposed by experts in the field in joint discussions with the FDA (1) including measurement of change in hepatic venous pressure gradient (HVPG), which is a measure of pressure in the main blood supply to the liver (viz., portal pressure). In the NASH-CX trial, we are using a reduction in HVPG as the primary endpoint for evaluating efficacy. Secondary endpoints in the study, some of which are also potential surrogate endpoints that have been discussed with the FDA, include liver biopsy evaluations of fibrosis, non-invasive diagnostic tests including FibroScan® to measure liver stiffness, which correlates with fibrosis, and the 13C-methacetin breath test (MBT, Exalenz Bioscience Ltd.) (2), which is a measure of liver metabolic function. Further secondary endpoints also include direct patient outcome measures related to complications of cirrhosis. We believe, based on our discussions with the FDA, that these endpoints will provide a broad and relevant set of measures to determine whether GR-MD-02 could be an effective therapy for NASH cirrhosis.
While a clinical trial protocol such as the NASH-CX trial is a detailed and complicated document (nearly 250 pages long in our case), the fundamentals are straightforward, as shown in the figure below. All patients enrolled do have cirrhosis and portal hypertension, but are well-compensated, meaning that they have not yet had complications related to their cirrhosis. Patients who meet the inclusion and exclusion criteria will be randomly allocated to receive either placebo or one of two doses of GR-MD-02. The trial is double-blinded, meaning neither the patients nor their physicians, nurses or Galectin Therapeutics will know what therapy they are receiving. The various diagnostic evaluations will be done as indicated in the figure and for each patient the changes in these evaluations at the end of treatment will be compared with the evaluations performed at the beginning of the study. The length of the study is related to the duration of GR-MD-02 treatment as well as the number of patients in the study to achieve a necessary sample size for scientific and statistical evaluations. For this study, a total of 156 patients will each receive either a dose of GR-MD-02 drug or placebo in biweekly infusions for one year.
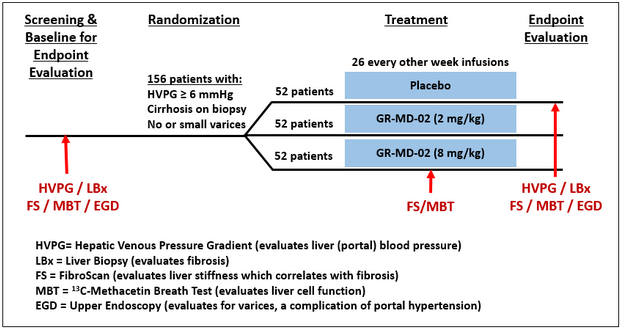
A number of investors have asked about the actual conduct of the clinical trial, so I will provide some details. We have engaged a large, multinational contract research organization (CRO) that is experienced in performing studies in liver disease, NASH, and cirrhosis. All study sites will be in the U.S. and will include a mix of large academic centers with internationally recognized clinical experts and prominent community-based health care centers. The two co-principal investigators for the trial, Dr. Naga Chalasani from Indiana University and Dr. Stephen Harrison from San Antonio Military Medical Center, are recognized experts in the field and were investigators in our Phase 1 clinical trial in NASH patients with advanced fibrosis.
Our most important operational milestone is the last patient randomized, in other words the first infusion of drug or placebo for patient number 156, which is targeted for August 2016. With a one-year treatment phase, if we meet this milestone then all patients will have completed therapy as of August 2017, with a goal for reporting top-line data by the end of 2017. Using the experience of our CRO in other trials of this type, we believe that we may achieve this goal with 45 study sites, but are able to increase that number to 60 study sites if necessary to stay on schedule. At this time, we have identified and pre-certified 55 study sites across the U.S., and are rapidly engaging to a total of 45 fully active study sites.
Fully establishing sites to enroll patients includes gaining approval from each of their investigational review boards (IRB), clinical and indemnification contracts, financial contracts, and education and training of their personnel. After sites are initiated, patients are screened in a process that involves performing multiple laboratory tests at different times, various invasive tests such as an upper endoscopy (EGD), liver biopsy and HVPG, and non-invasive tests such as FibroScan and MBT; this testing alone may take up to eight weeks. Assuming a patient passes the various screening parameters for entry into the trial from all these tests, they are then randomized and begin receiving bi-weekly infusions of drug or placebo. Sites are currently screening, enrolling and treating patients.
What do we expect to learn from this study? First, we expect to learn whether GR-MD-02 can reduce the portal pressure in a patient population of well-compensated cirrhosis, a measure that is known to be directly related to complications of cirrhosis and patient mortality. Second, we expect to learn whether our drug reduces portal pressure by reducing the amount of scarring in the liver as determined by liver biopsy. Finally, we anticipate learning how a reduction in fibrosis and portal pressure correlates with non-invasive testing such as FibroScan, MBT, serum biomarkers and other tests including a quality-of-life questionnaire and complications of cirrhosis. The NASH-CX trial is a rigorously designed study to gain the most information possible regarding the effect of GR-MD-02 in these patients, who have no current available therapy. The NASH-CX trial also importantly serves to help design later-stage trials such as those that may be required for regulatory approval. With success in this trial, GR-MD-02 could be the first drug shown to reduce fibrosis in cirrhotic patients and the first hope of a treatment in this patient population other than liver transplant.
More information on Galectin’s NASH-CX trial can be found at clinicaltrials.gov
- NASH-CX: A Multicenter, Randomized, Placebo-controlled, Double-blind, Parallel-group, Phase 2 Clinical Trial to Evaluate the Safety and Efficacy of GR-MD-02 for the Treatment of Liver Fibrosis and Resultant Portal Hypertension in Patients With Nash Cirrhosis https://clinicaltrials.gov/ct2/show/NCT02462967?term=GR-MD-02&rank=3
These “CEO Perspectives” will be a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “could,” “expect” and others. These statements include those regarding the timing of enrollment of patients and the hope that Galectin’s development program for GR-MD-02 will lead to the first therapy for the treatment of fatty liver disease with advanced fibrosis and cirrhosis. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements
Reference List
1. Sanyal AJ, Friedman SL, McCullough AJ, Dimick-Santos L. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: findings and recommendations from an American Association for the Study of Liver Diseases-U.S. Food and Drug Administration Joint Workshop. Hepatology 2015 Apr;61(4):1392-1405.
2. Exalenz Bioscience Ltd. (Israel). See www.exalenz.com for information on Exalenz and the 13C-methacetin breath test
Make a Comment or Ask a Question
[contact-form-7]The post Clinical Trial to Establish Efficacy of GR-MD-02 in NASH Cirrhosis appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Clinical Development Program in Liver Fibrosis appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>Galectin Therapeutics is developing a drug called GR-MD-02 which binds to and inhibits the galectin-3 protein which is important in the promotion of multiple types of disease including inflammation, fibrosis, and cancer. We have focused the use of this drug in a development program for potential treatment of patients with liver fibrosis, and specifically in those patients with NASH (non-alcoholic steatohepatitis) with advanced fibrosis or the most severe form of fibrosis, cirrhosis. There are currently no available therapies for liver fibrosis due to NASH.
A clinical development program includes a complex set of scientific experiments and human clinical trials that hopefully results in marketing approval of a drug for use in patients. One of the most important aspects of a clinical development program is specifying the indication, which guides all other decisions. In our case, we are focused on the treatment of NASH in two key target populations, NASH with cirrhosis, and NASH with advanced fibrosis, but not cirrhosis. The importance of the unmet medical need in these patients and the strength of the data from our preclinical studies with GR-MD-02 resulted in the FDA giving this development program Fast Track status. Our overall drug development program includes studies in all of the following areas:
- Pre-clinical (animal) studies to show efficacy in disease models
- Pre-clinical (animal) studies to evaluate for potential drug toxicity
- Pre-clinical (animal) studies to evaluate how the drug is distributed in the body, metabolized, and eliminated from the body
- Chemical development studies to characterize the structure and quality of the chemical or new molecular entity
- Process development studies to design and optimize the production processes
- Pharmaceutical or formulation development studies to design an appropriate form and route of administration for the final drug product
- Clinical Phase 1 studies to demonstrate safety in small numbers of healthy volunteers and patients with NASH with advanced fibrosis
- Clinical Phase 2 studies to establish efficacy and dose, as well as safety in the indicated patient population
- Clinical Phase 3 studies to confirm safety and efficacy for the intended marketing indication and target patient population
A great deal of work has already been completed on the development program for GR-MD-02. Pre-clinical animal studies have been completed that demonstrate efficacy in two different liver disease models and this work has been published in the peer-reviewed scientific literature (1, 2). Multiple studies have been done in animals to evaluate potential toxicity of GR-MD-02 and how the drug is handled in the body. Extensive work has been performed to establish the pharmaceutical quality of the drug and production processes. Several batches of consistent quality have been produced in accordance with FDA requirements for human use and administration. These studies provided the foundation for the FDA allowing us to move into human trials.
Human studies are divided into three phases. Phase 1 studies refer to the first human studies and are primarily to determine drug safety in a small number of patients, usually healthy volunteers. However, some Phase 1 trials are allowed to be performed in diseased patients in order to also evaluate whether the drug is having the intended effect. Phase 2 studies focus on evaluation of efficacy as well as safety of the drug in subjects with the target disease. This is the phase of clinical development when it is determined whether the drug doses studied may be effective in the disease indication. Phase 3 trials are larger studies that confirm the efficacy of the drug, evaluate safety in a larger patient population, and serve as the primary registration trials for marketing approval.
Galectin has completed two Phase 1 clinical trials, with one in NASH patients with advanced fibrosis and one in healthy volunteers. The Phase 1 trial in patients with NASH fibrosis was highly successful demonstrating safety, providing dosing information for phase 2, and showing an effect on markers of fibrosis. This will be the subject of its own “CEO Perspective” in the future. On the basis of these Phase 1 studies, Galectin has initiated two phase 2 clinical trials to evaluate the possible reversal of liver fibrosis in NASH, the most common form of liver disease. The NASH-CX trial will focus on reversal of fibrosis in the most severe form of fibrosis called cirrhosis and the NASH-FX trial will attempt to show reversal of advanced fibrosis in a pre-cirrhotic stage.
More information on Galectin’s clinical trials can be found at clinicaltrials.gov
- Phase 1 https://clinicaltrials.gov/ct2/show/NCT01899859?term=GR-MD-02&rank=2
- NASH-CX: https://clinicaltrials.gov/ct2/show/NCT02462967?term=GR-MD-02&rank=3
- NASH-FX: https://clinicaltrials.gov/ct2/show/NCT02421094?term=GR-MD-02&rank=4
These “CEO Perspectives” will be a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may”, “could”, “expect” and others. These statements include those regarding the hope that Galectin’s development program for GR-MD-02 will lead to the first therapy for the treatment of fatty liver disease with advanced fibrosis and cirrhosis. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements
Please look for future editions in which multiple aspects of our development programs for unmet medical needs will be addressed.
Reference List
- Traber PG, Chou H, Zomer E, Hong F, Klyosov A, Fiel MI, et al. Regression of fibrosis and reversal of cirrhosis in rats by galectin inhibitors in thioacetamide-induced liver disease. PLoS One 2013;8(10):e75361.
- Traber PG, Zomer E. Therapy of experimental NASH and fibrosis with galectin inhibitors. PLoS One 2013;8(12):e83481.
Make a Comment or Ask a Question
[contact-form-7]The post Clinical Development Program in Liver Fibrosis appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>