By Peter G. Traber, M.D. on December 7, 2015
In June 2015 Galectin Therapeutics initiated a clinical trial to determine if our investigational drug GR-MD-02 can successfully treat patients with severe scarring of the liver (cirrhosis) due to fatty liver disease (a.k.a. nonalcoholic steatohepatitis, or NASH). The goal of the study (the NASH-CX trial) is to assess whether GR-MD-02 may reduce the fibrous tissue that is clogging the liver in subjects with NASH cirrhosis. A reduction, in turn, is expected to improve liver function and have a positive effect on patient outcomes, such as perhaps delaying or avoiding a liver transplant. Please see my earlier CEO Perspective, “Clinical Trial to Establish Efficacy of GR-MD-02 in NASH Cirrhosis,” where this study is described in more detail.
Can you participate in this clinical trial?
If your cirrhosis is caused by NASH and you have portal hypertension, but you haven’t developed other complications related to your cirrhosis, then you might be eligible to participate in this trial. You must be between 18 and 75 years old and meet various other study eligibility requirements. This study is limited to people with cirrhosis only caused by NASH and not by any other factors, like alcohol or hepatitis.
If you qualify, you would be one of 156 participants at about 45 to 60 study sites, or clinics, across the United States. It is important to remember that this is an experimental study comparing GR-MD-02 against a placebo; the trial design is such that you have 2 out of 3 chances of being on active drug and 1 out of 3 chances of being treated with placebo.
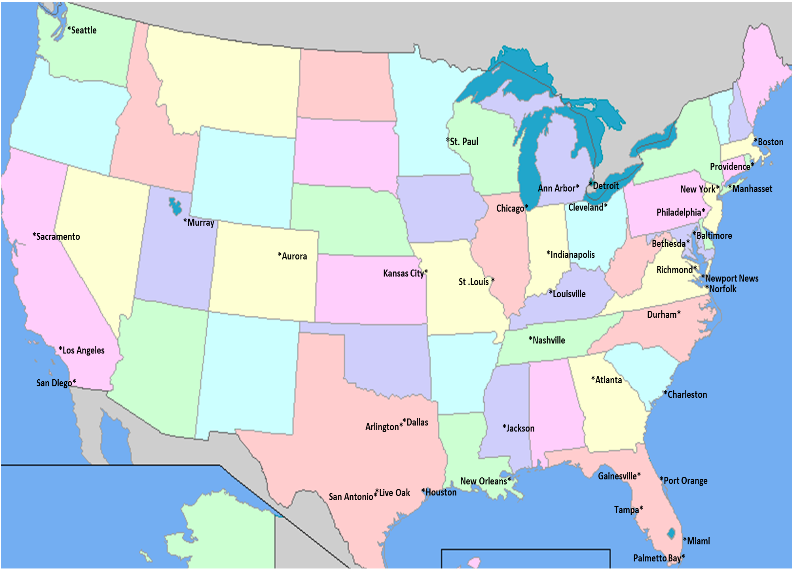
The study medication will be administered by an intravenous infusion every two weeks for one year. We expect that each clinic visit will last about an hour and a half. Below you will find a map showing the cities where the study is being conducted to see if you are able to make it to the site every two weeks. If you think you might be eligible for our study, please contact one of the study sites (click here for the list of sites and the appropriate contact information).
More information about this trial can be found at clinicaltrials.gov:
• NASH-CX: https://clinicaltrials.gov/ct2/show/NCT02462967?term=GR-MD-02&rank=3
Should the NASH-CX trial be successful, this will be the first clinical study of an investigational drug candidate to show a reduction of fibrosis in people with cirrhosis and the first hope of a future approved treatment other than a liver transplant.
These “CEO Perspectives” will be a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “could,” “expect” and others. These statements include those regarding the hope that Galectin’s development program for GR-MD-02 will lead to the first therapy for the treatment of fatty liver disease with advanced fibrosis and cirrhosis. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements
Please look for future editions in which multiple aspects of our development programs for unmet medical needs will be addressed.
GT-026 STUDY SITE MAP