The post Photographs of Improvement in Moderate-To-Severe Plaque Psoriasis appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>Patients treated with GR-MD-02 experienced significant clinical improvement
As described in the press release, each of the four patients who received six doses of GR-MD-02 over 12 weeks of therapy reported improvement in their psoriasis symptoms. Moreover, three patients had significant, and one had modest, improvement in the Psoriasis Area Severity Index (PASI), which is an objective and standardized means of evaluating disease response (see table below).
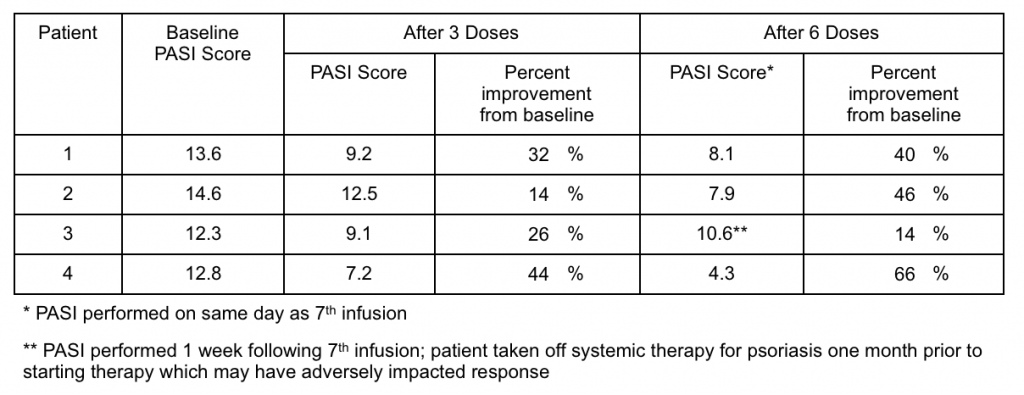
In addition to these quantitative results, photographs provide vivid evidence of response to therapy (below). Each patient had over 70 pictures taken at each photography session, so this represents only a small sample and not a full representation of their whole body disease. These photographs provide evidence of disease improvement in areas of significant improvement.
Patient 1 had complete clearing of multiple chest lesions. Patient 2 had near complete resolution of arm and elbow lesions. Patient 4, the one with the greatest reduction in PASI score, had nearly complete resolution of an abdominal lesion and remarkable improvement of very severe leg lesions. Most of the disease on Patient 4’s leg is actually completely resolved with only some skin hyperpigmentation, which generally takes months to slowly fade.
What does this mean for our drug GR-MD-02?
It is uncommon for patients with moderate-to-severe plaque psoriasis to spontaneously improve without treatment, so the improvements we have seen in this trial are due to GR-MD-02. Furthermore, these improvements are clinically significant for these patients, as all of them suffered from moderate-to-severe psoriasis for years (between 6 and 40 years) prior to entering the trial. Therefore, it is our belief that GR-MD-02 had a positive effect on psoriasis in these patients.
We believe this is the first definitive clinical effect of GR-MD-02 to be shown in patients. While all diseases are not the same in terms of etiology, these results support our optimism for GR-MD-02 to affect other disease processes that the company is investigating.
How do our results with GR-MD-02 compare with other available psoriasis therapies?
Beyond the fact that GR-MD-02 has a biological effect on psoriasis in patients, we need to consider the possibility that GR-MD-02 could be additive to the medical armamentarium for treating psoriasis. There are multiple commercial and development-stage drugs for the treatment of moderate-to-severe plaque psoriasis, including biological agents that would constitute the direct competition for GR-MD-02.
There is an established FDA regulatory pathway for drug approval in moderate-to-severe plaque psoriasis that all the recent marketed biological drugs have followed. PASI is universally used in registration clinical trials (also referred to as phase 3 or pivotal clinical trials) as an objective measurement of drug response. All registration clinical trials assess the number of patients who achieve a 75% improvement in PASI (called PASI-75) at various times following initiation of therapy. For currently approved drugs, the percentage of patients who achieve PASI-75 at between 12 and 24 weeks of therapy ranges from 54% to as high as 90% in some studies.
At the time of this interim analysis of our trial, none of the four patients had reached a PASI-75, which is why we have taken the next steps described below.
What are the next steps?
We have extended therapy in the study with the current dose of GR-MD-02 for a full 24 weeks, or 13 infusions, to determine whether improvement at this dose continues and reaches PASI-75. Extension beyond 24 weeks of therapy to reach PASI-75 would not be competitive from an efficacy standpoint among the currently marketed biological agents for moderate-to-severe plaque psoriasis. However, in general, approval of drugs is a balance between efficacy and safety and most of the drugs on the market have serious side effect profiles. We are now attempting to enroll the full cohort of 10 patients in this trial, with all receiving 24 weeks of therapy. We will continue to report interim data from this trial.
This interim analysis of four patients has given us great encouragement on the biological effect of GR-MD-02 in a human disease and we look forward to reporting results later this year on this trial and our trial with GR-MD-02 in patients with NASH and advanced (stage 3) fibrosis (the NASH-FX trial).
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including whether GR-MD-02 may be effective in the treatment of psoriasis. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2015, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Make a Comment or Ask a Question
[contact-form-7]The post Photographs of Improvement in Moderate-To-Severe Plaque Psoriasis appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post How Will We Interpret Results from the GR-MD- 02 Psoriasis Trial? appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>Last September, Galectin Therapeutics began a Phase 2a open-label clinical trial with GR-MD-02, our galectin-3 inhibitor drug, in patients with moderate-to-severe plaque psoriasis. We initiated this trial because a patient in our Phase 1 trial with GR-MD-02 in NASH had a remarkable and sustained remission of her psoriasis following treatment, as described in a previous CEO Perspective (here). Since then, I have been asked on numerous occasions how we will interpret the results of this trial.
What are the psoriasis trial endpoints?
Multiple drugs have been approved for the treatment of psoriasis, so there is an established FDA regulatory pathway to approval. It is well accepted that assessment of drug efficacy in psoriasis clinical trials is best performed using the Psoriasis Area and Severity Index, or PASI. PASI is used both to measure the severity of psoriasis as well as to measure the effect of treatment. While this index score is not generally used in the clinical practices of dermatology, it is universally used in clinical trials as an objective measurement of drug response.
PASI is an objective set of measurements that are conducted by a physician to assess the area of skin involvement and the severity of skin lesions in patients with psoriasis. The figure below, taken from a review article written by experts in the field, outlines the approach to the measurement (1).
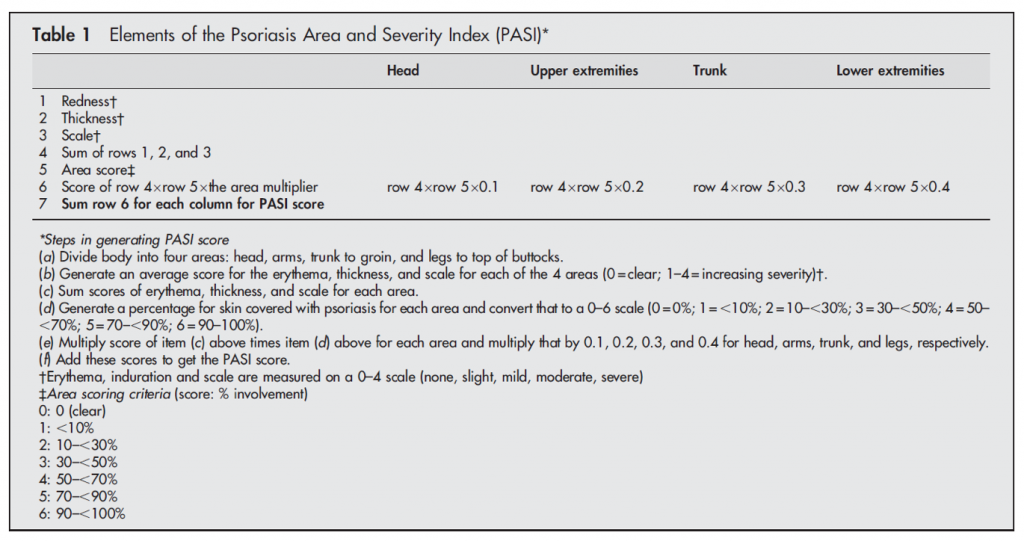
Therefore, the primary endpoint in our psoriasis trial will focus on improvement in PASI following treatment with GR-MD-02. We will evaluate the number of patients who have various percentage improvements in PASI. We will also record the durability of response to treatment for up to one year, should there be a beneficial effect. Finally, we are monitoring the incidence of adverse events and vital sign and laboratory abnormalities, if any, during study treatment.
What constitutes a meaningful effect?
Moderate psoriasis is generally defined as a PASI of ≥10. In our trial, as is typical for clinical trials in moderate-to-severe psoriasis, patients who enroll in the trial are required to have at least 10% of their body area affected by psoriasis and a PASI of ≥12, parameters which were agreed to with the FDA. Moreover, the patients will have the diagnosis of psoriasis confirmed by a skin biopsy. Therefore, patients in the trial will have significant disease and are similar to those who have been enrolled in the trials of approved drugs for psoriasis.
Once an individual has established psoriasis, it generally does not spontaneously dissipate or improve. As published in the medical literature, it has been suggested that clinically significant improvements in psoriasis are clearly obtained when PASI is lowered by 50% of baseline values, i.e., a PASI 50 response (2). However, the accepted clinical trial endpoint for drug registration is PASI 75, meaning a 75% reduction in psoriasis versus baseline. However, since this is an exploratory trial in which we do not have any information on optimal dosing, we will be looking for any objective response.
What will this trial tell us and what might be the next steps?
First and most important, our exploratory psoriasis clinical trial will either confirm the effect on psoriasis seen in the one Phase 1 patient, or show that her remission was unrelated to our drug. Should a significant proportion of patients in the trial respond to GR-MD-02 with an improvement in their psoriasis, such a finding would represent the first human disease response to GR-MD-02. That would be an important finding with potential implications for activity in our main therapeutic program for NASH, a disease where there is a higher incidence of psoriasis.
Second, this trial may indicate whether GR-MD-02 is a viable candidate to advance as a therapy for psoriasis. There are a number of biologic agents that have been approved in recent years for moderate-to-severe psoriasis, and GR-MD-02 would need to be competitive with those drugs. In my view, based on the efficacy of approved drugs, GR-MD-02 would need to demonstrate a PASI 75 in at least 50% of treated patients to consider full clinical development. If it does have such a degree of efficacy, it might be considered as a potential drug therapy on the basis of its lower cost to manufacture than existing drugs, a potentially better safety profile or the duration of effect after stopping treatment.
While each of these potential benefits would need to be studied in detail, the first step is to determine whether the drug has an effect on the disease, which is the primary goal of this study.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including whether GR-MD-02 may be effective in the treatment of psoriasis. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2015, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Reference List
1. Feldman SR, Krueger GG. Psoriasis assessment tools in clinical trials. Ann Rheum Dis 2005 Mar;64 Suppl 2:ii65-ii68.
2. Carlin CS, Feldman SR, Krueger JG, Menter A, Krueger GG. A 50% reduction in the Psoriasis Area and Severity Index (PASI 50) is a clinically significant endpoint in the assessment of psoriasis. J Am Acad Dermatol 2004 Jun;50(6):859-866.
Make a Comment or Ask a Question
[contact-form-7]The post How Will We Interpret Results from the GR-MD- 02 Psoriasis Trial? appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post 2015 – 2016, Progress and Possibilities appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The many accomplishments at Galectin Therapeutics during 2015 ranged from incremental progress touching every aspect of our business to significant advancements with GR-MD-02. Importantly, this progress forms the basis for numerous milestones expected in the coming years including clinical progress, intellectual property fortification, further engagement with the investment community and ongoing outreach to educate our shareholders about our work to develop new therapies, the regulatory environment in which we operate and our target markets.
CEO Perspectives, Dr. Traber’s blog introduced last year, is designed to provide scientific and technical information largely regarding our work with GR-MD-02 in layman’s language. Much of our progress and accomplishments during 2015 were chronicled in the 14 blog postings, which can be found here.
We were delighted that in the final days of 2015 a U.S. District Court dismissed both the federal securities class-action lawsuit and the shareholder derivative actions lawsuit filed in the Summer of 2014 against Galectin and certain officers, directors and a shareholder, which had cast an inappropriate cloud over our many achievements in 2015. The Court entered final judgments of dismissals in both actions based on the Court’s finding that any further amendment of the complaints would be futile (i.e., dismissed with prejudice). Plaintiffs have the right to appeal the Court’s dismissals within 30 days. Based on the Federal Court’s rulings, Galectin is seeking dismissal of a duplicative shareholder derivative action in Nevada which was filed after the federal actions.
Our Clinical Programs
NASH with advanced liver fibrosis
Development of GR-MD-02 for the treatment of non-alcoholic steatohepatitis (NASH) with advanced fibrosis and cirrhosis continues to be the primary focus of our company. We completed a successful Phase 1 clinical trial and announced final data in January 2015. The Phase 1 trial demonstrated that GR-MD-02 is safe, with potential for therapeutic effect on fibrosis in NASH patients with advanced fibrosis. We found no serious adverse events and no treatment-emergent adverse events related to our drug. Furthermore, GR-MD-02 was found to be safe and well tolerated in each of the three dose-escalating cohorts of patients, who were suffering from NASH with advanced fibrosis.
This finding alone defines the study as a success, but we gained additional valuable information. We found that the FibroTest® score, a composite biomarker of five different blood tests that has been correlated with the extent of liver fibrosis, was significantly reduced by GR-MD-02 treatment in the third dosing cohort of 8 mg/kg. In addition, we found that some patients in this cohort also showed a decrease in liver stiffness, which has a direct correlation with fibrosis. We published a comprehensive piece on the results of the Phase 1 study in a CEO Perspectives blog post, which can be found here.
In addition to the phase 1 trial in NASH patients with advanced fibrosis, we reported the results of a drug-drug interaction study with GR-MD-02 and midazolam, a common sedative, which showed that in healthy volunteers there was no unfavorable interaction between the two compounds. Because many patients with chronic diseases are on multiple medications over long periods of time and may take other medications on an intermittent basis, this finding is important to the commercial potential of GR-MD-02 and to the patient population that is eligible to participate in our Phase 2 program. We published a CEO Perspectives piece on this topic, which is available here.
The information gleaned from the Phase 1 studies formed the basis for our Phase 2 program, which consists of two studies, one in NASH patients with advanced fibrosis and the other in NASH patients with cirrhosis. We submitted our protocol to the U.S. Food and Drug Administration (FDA) for the cirrhosis study in the first quarter, engaged our contract research organization and began screening patients at the end of June.
This study, the NASH-CX trial, is a multicenter, randomized, placebo-controlled, double-blind, parallel-group Phase 2 trial to evaluate the safety and efficacy of GR-MD-02 for the treatment of liver fibrosis and resultant portal hypertension (HVPG) in patients with NASH cirrhosis. A total of 156 patients at approximately 50 sites in the U.S. will be randomized to receive either 2 mg/kg of GR-MD-02, 8 mg/kg of GR-MD-02 or placebo, with 52 patients in each arm. The primary endpoint is a reduction in HVPG. Patients will receive a total of 26 infusions every other week for one year, at which time they will be evaluated for change in HVPG compared with placebo. HVPG will be correlated with secondary endpoints of fibrosis on liver biopsy as well as with measurement of liver stiffness via FibroScan® and assessment of liver metabolism (13C-methacetin breath test, Exalenz), which are non-invasive measures of the liver that may be used in future studies. More information can be found at www.clinicaltrials.gov and in a CEO Perspectives blog post, which can be found here.
We are pleased with the pace of the NASH-CX study and we remain on track to provide data readout in at the end of 2017, as we have previously indicated.
In September we initiated a 30-patient study with GR-MD-02 in NASH patients with advanced fibrosis, our NASH-FX study, with 15 patients receiving 8 mg/kg of GR-MD-02 and 15 patients receiving placebo every other week for 16 weeks. This study will evaluate the safety and efficacy of GR-MD-02 on liver fibrosis using multi-parametric magnetic resonance imaging (LiverMultiScan®, Perspectum Diagnostics) as the primary endpoint and liver stiffness as assessed by magnetic resonance-elastography and FibroScan as secondary endpoints. This study is also proceeding as planned, with top-line data expected around the end of the third quarter of 2016. We published a CEO Perspectives piece on this trial, which is available here.
Psoriasis
As we have previously reported, one of the patients participating in our Phase 1 NASH study was a long-term psoriasis sufferer, and this patient’s psoriasis cleared as the study progressed, and remained cleared for many months following the conclusion of the study. With an established theoretical pathway for how inhibition of galectin-3 might affect psoriasis, in September we began an open label 10-patient Phase 2a pilot study in patients with moderate-to-severe plaque psoriasis. We expect data readout from this study late in the third quarter of 2016. More information and background on this study can be found here.
Melanoma
We continued to support independent research with GR-MD-02 in combination with two commercial melanoma drugs, as preclinical research has shown our compound enhances the efficacy of immune checkpoint blockade therapies, or so-called checkpoint inhibitors, a new class of drugs. GR-MD-02 is progressing through a Phase 1b study in combination with Yervoy®, and a Phase 1b study in combination with Keytruda® was initiated in the fourth quarter of 2015 with enrollment to begin early in 2016. Preclinical work in mouse cancer models with GR-MD-02 added to checkpoint inhibitors shows a boost in anti-tumor immunity, a reduction in tumor size and increased survival. Both of these studies are being conducted at the Providence Cancer Center in Portland, Oregon. Galectin is providing GR-MD-02 to the investigators, who are funding the costs of these studies. We published a CEO Perspectives on these trials, which is available here.
In the trial combining Yervoy and GR-MD-02, two dosing cohorts have been completed and the third cohort delivering 4 mg/kg of GR-MD-02 is enrolling now. Of the seven patients that have received the combination therapy, there has been no dose limiting toxicity. Following completion of the 4 mg/kg dose cohort, a total of 10 patients will be dosed at 8 mg/kg. Immune markers as well as tumor response are being monitored in this study.
Significant Presentations and Publications
Galectin’s researchers presented at several important industry meetings during the year. Dr. Traber delivered an invited presentation of the company’s research with GR-MD-02 in NASH at the American Association for the Study of Liver Diseases (AASLD) Industry Colloquium in March. He participated in the session entitled “NASH: Clinical Endpoints and Drug Development” and discussed the role of galectin-3 in organ fibrosis generally and liver fibrosis in particular, and GR-MD-02 as a galectin-3 inhibitor. He reviewed the published preclinical data showing that GR-MD-02 is effective in reversing inflammation and fibrosis in a mouse model of NASH and also in reversing cirrhosis and improving portal hypertension in a rat model of cirrhosis. He also reviewed the company’s Phase 1 study results and its Phase 2 clinical program design. The abstract of Dr. Traber’s presentation can be found here.
In addition, preclinical research from a study led by Stefanie Linch, Ph.D. in the laboratory of tumor immunology expert William L. Redmond, Ph.D. of the Providence Cancer Center’s Earle A. Chiles Research Institute was presented in November at the Society for Immunotherapy of Cancer’s (SITC) 30th Anniversary Annual Meeting. The studies presented were conducted by the Institute in collaboration with Galectin Therapeutics. The poster presentation “Galectin-3 inhibition using novel inhibitor GR-MD-02 improves survival and immune function while reducing tumor vasculature” and an abstract was published in the Journal for ImmunoTherapy of Cancer. The poster presentation is available for review here.
Interviews with Dr. Traber appeared in a number of publications throughout 2015, including R&D Magazine in a piece entitled “Finding the Holy Grail Treatment for Fatty Livers,” available here, Obesity News Today published its Q&A article entitled, “Exclusive: Dr. Peter Traber Discusses Non-alcoholic Fatty Liver Disease”, available here, and MD Magazine, which conducted an online interview with Dr. Traber at the AASLD meeting. That interview can be found here.
Galectin management also participated in a number of investment conferences throughout the year, including programs for institutional investors, retail investors and family offices.
Foundational Support for our Business
During 2015 we considerably strengthened our intellectual property portfolio and received a U.S. patent Notice of Allowance for the use of pectin compounds to reduce fibrosis in multiple diseases. This patent is particularly important because it not only permits GR-MD-02 use for NASH with fibrosis, but it covers other compounds in our pipeline and a multitude of diseases with a fibrotic etiology. We also continued to build our international patent portfolio with patents issued or allowed in Israel and Australia.
We also made excellent progress with our Chemistry, Manufacturing and Controls (CMC), all of which are vital to the proper conduct of our clinical trials with GR-MD-02 and are essential components of the final application to the FDA for a drug’s approval. We discussed this progress during the year in the CEO Perspective post found here. We also reached a very significant milestone in our preclinical toxicology program, having completed chronic administration of GR-MD-02 in two animal species, allowing chronic administration in human subjects.
Lastly, we were very pleased to have completed a $9.8 million financing during the fourth quarter. This capital is expected to fund currently planned operations through the first quarter of 2017, and will be used mainly for clinical trial expenses and other research and development expenses, as well as for general corporate purposes.
Looking Ahead to 2016
We are looking forward to executing on several important milestones in 2016, with highlights including the following:
- We will continue enrollment in the NASH-CX study and work with our investigators and contract research organization to keep on our stated timelines for data readout in late 2017
- We will continue enrollment in the NASH-FX study, and continue to expect data readout around the end of the third quarter of 2016
- We will continue enrollment in the psoriasis Phase 2a study, with data readout also expected at the end of the third quarter of 2016
- While we do not control the rate of enrollment of the trial, we expect data from the Providence Cancer Center’s study with Yervoy in combination with GR-MD-02 in advanced metastatic melanoma by the end of 2016.
- We expect that Providence Cancer Center will be enrolling patients in the study with GR-MD-02 in combination with Keytruda during 2016.
We are fully aware that yesterday’s accomplishments set tomorrow’s expectations, and we look forward to creating shareholder value by executing on numerous milestones during 2016. We are grateful to our long-standing, loyal shareholders for their continued support and to the hard-working staff at Galectin Therapeutics who share a unifying commitment to addressing significant unmet clinical needs in NASH, as well as in oncology and psoriasis.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. These statements include those regarding the hope that Galectin Therapeutic’ s development program for GR-MD-02 will show that it can be both safe and effective when used in combination with other drugs for the treatment of patients with cancer. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements
Make a Comment or Ask a Question
[contact-form-7]The post 2015 – 2016, Progress and Possibilities appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Exploratory Indication in Psoriasis appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>Serendipity, or fortunate happenstance, has always been an important part of the search for medicines. As pointed out by Thomas A. Ban (1), serendipity in drug discovery is more than just luck, but rather is best described by Pasteur’s famous quote that “Chance favors the prepared mind”. Serendipity may have entered our development program with our anti-galectin drug, GR-MD-02, and today we kick off another Phase 2 clinical trial to test whether an unexpected effect observed in our Phase 1 trial is truly something of significance.
Our surprising clinical trial observation
In the course of our Phase 1 clinical trial of GR-MD-02 in patients with fatty liver disease (NASH) and advanced fibrosis, we encountered a patient with plaque psoriasis, who happened to be one of the patients treated with four infusions of 4 mg/kg of GR-MD-02. Psoriasis is a relatively common, chronic skin disorder that causes a red, thickened and scaling rash that can cover many parts of the body.
Upon the patient’s follow-up visit to her physician, she reported resolution of her long-standing psoriasis. In an interview with a dermatologist and myself, the patient confirmed that she had severe psoriasis involving much of both arms continuously for six years. Following treatment with GR-MD-02, the psoriasis resolved and has not recurred for over a year! There was another patient in the study with mild psoriasis who was able to reduce use of topical steroids and another with severe psoriasis who had just been withdrawn from therapy with Stelara (ustekinumab), a powerful injection therapy for psoriasis, who did not improve.
Observation prompts exploratory clinical trial
On the strength of the marked effect in the one patient, both a dermatologist investigator and we felt it was worth investigating whether this was a real effect of GR-MD-02. In addition to the clinical finding, research publications suggest the potential importance of galectin-3 in psoriasis (2, 3). Therefore, we initiated a phase 2a, open label clinical trial in patients with moderate to severe plaque psoriasis (press release September 22, 2015). In this trial, 10 psoriasis patients with ≥ 10% of their skin affected and a PASI (psoriasis activity and severity index) of ≥ 12 points will be treated with 7 every other week infusions of 8 mg/kg GR-MD-02. The severity of psoriasis was agreed upon with the FDA as warranting experimental therapy. More information on the trial can be found at: https://clinicaltrials.gov/ct2/show/NCT02407041?term=GR-MD-02&rank=5
The primary endpoint for evaluation of these patients will be the PASI-75, which means that they have had a 75% improvement in the severity of the disease 30 days following the final infusion. This is a standard endpoint used in studies of drugs that have been approved for marketing. The PASI system assesses each affected body area as to the extent of skin redness, thickness, and scaling. We intend on reporting data 30 days following treatment of the last patient treated. Additional results would then be reported following 6 month and 1 year follow up periods to assess for durability of response.
What is next following exploratory trial?
Our current trial is based on an observation with one patient. It is promising enough to investigate in an exploratory trial, but single-patient observations are notoriously unreliable. Therefore, while we anticipate results, we are cautious in our optimism.
We will consider this clinical trial successful if ≥ 50% of patients treated meet the primary efficacy endpoint. Positive results from this trial would be significant, since we would have demonstrated a potentially important clinical effect of our galectin-3 inhibiting drug, GR-MD-02. Moreover, it is of interest that there is a relatively high incidence of NASH in patients with psoriasis (4, 5), which is our other major indication for GR-MD-02.
With positive results, we will then consider entering into a development program aimed at marketing approval for psoriasis. There are already multiple excellent drugs on the market for this indication, some of which have been approved in the last few years. Potential areas that GR-MD-02 may be differentiated from other drugs include safety, prolonged response (if the one patient results are confirmed), and lower cost manufacture.
These “CEO Perspectives” will be a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “could,” “expect” and others. These statements include those regarding the hope that Galectin’s development program for GR-MD-02 will lead to the first therapy for the treatment of fatty liver disease with advanced fibrosis and cirrhosis. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements
Please look for future editions in which multiple aspects of our development programs for unmet medical needs will be addressed.
Reference List
1. Ban TA. The role of serendipity in drug discovery. Dialogues Clin Neurosci 2006;8(3):335-344.
2. Chen HY, Lo C-H, Li C-S, Hsu DK, Liu FT. Galectins and cutaneous immunity. Dermatologica Sinica 2012;30:121-127.
3. Lacina L, Plzakova Z, Smetana K, Jr., Stork J, Kaltner H, Andre S. Glycophenotype of psoriatic skin. Folia Biol (Praha) 2006;52(1-2):10-15.
4. Roberts KK, Cochet AE, Lamb PB, Brown PJ, Battafarano DF, Brunt EM, et al. The prevalence of NAFLD and NASH among patients with psoriasis in a tertiary care dermatology and rheumatology clinic. Aliment Pharmacol Ther 2015 Feb;41(3):293-300.
5. Wenk KS, Arrington KC, Ehrlich A. Psoriasis and non-alcoholic fatty liver disease. J Eur Acad Dermatol Venereol 2011 Apr;25(4):383-391.
Make a Comment or Ask a Question
[contact-form-7]The post Exploratory Indication in Psoriasis appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>