The post The Purpose of the CEO Perspectives Blog appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>First and foremost, the blog provides a mechanism for more detailed and economical communication with multiple groups and constituencies. It allows discussion of issues and expansion of information that is not appropriate for, or is constrained by, other outlets such as press releases and formal presentations. The posts are published to the Web, emailed to list of those whom have signed up for receiving company information, and distributed on social media including LinkedIn, Twitter, and Facebook. The goal is to inform and attract a broad readership.
As a company, we want to be as transparent about our activities as possible, and we want to use cutting-edge methods of communication to do so.
The content of the blog is intended to address direct programs of Galectin Therapeutics, general areas of importance for fatty liver disease (including liver fibrosis and cirrhosis), and the importance of galectin proteins in various diseases. In concert with regular press releases and meeting presentations, CEO Perspectives provides content that can be distributed to many different types of audiences. It is important to note, however, that press releases and SEC regulatory filings remain the only routes for communicating material company information. While I have at times published posts that complement press releases, the blog serves to supplement official communications from the company, not replace them.
The audiences that we intend to reach through the blog include current shareholders, prospective shareholders and investors, industry analysts, pharma and biotech companies, patients and advocacy groups, and the industry and popular press.
One of the main drivers behind CEO Perspectives is to help investors understand the tremendous value of what Galectin Therapeutics is doing in developing potential treatments for NASH cirrhosis and other diseases. The value of the company over the next year rests on success of our ongoing programs, which were outlined in various press releases and include:
- Data from the NASH-CX trial in patients with NASH cirrhosis which is completely enrolled and is scheduled to report top line data in December 2017. (see recent press release)
- The possibility of developing pharma partnerships for treating psoriasis and atopic dermatitis based on our exciting results of clinical effects in an exploratory clinical trial. (see recent press release)
- Combination immunotherapy trials being performed at Providence Cancer Center which will have interim data reported at Immunotherapeutics & Immunomonitoring Conference, to be held on February 6-7, 2017, in San Diego, California. (see recent press release)
For our shareholders and prospective investors, the posts are meant to supplement information in press releases and conference presentations. Sometimes they will be directly relevant to the value creation programs above, and sometimes not. I continue to value the feedback I get from investors about the blog, but other audiences are also important to the company.
For example, multiple industry analysts and pharma companies have found the blog useful in assessing our technology and programs. Many of the patients who are in our clinical trials or who have relevant disorders, as well as patient advocacy groups, have indicated the blog is helpful for their education.
The press is another important audience for the blog. The blog posts have proven useful to educate and inform reporters, which has led to boarder coverage of the company. Some examples of these results are the articles in the following industry and popular press outlets:
- Pharmacy Times—“Nonalcoholic Steatohepatitis Drug Pipeline Overview”
- HealthZette—“The New Liver Killer: Silent, Sneaky, Deadly”
- Drug Discovery & Development—“Why Non-Invasive Testing is Needed for Liver Fibrosis”
- Newsweek—“NASH is the 21st century’s looming public health threat”
- R&D Magazine—“Finding the “Holy Grail” Treatment for Fatty Livers”
The CEO Perspectives blog is meant to be informative for multiple audiences, to serve as content to expand the knowledge and understanding of Galectin Therapeutics’ programs, to bring more people to an understanding of the potential of our programs, and to educate people about the medical science that touches on our ultimate success. The path to an interest in the company can take multiple paths, and the blog helps to facilitate a number of them. It is not the only component of our outreach, but it has served as a useful mechanism for communication. I hope that all our audiences continue to find the posts useful.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including whether GR-MD-02 may be effective in the treatment of NASH and various other diseases. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2015, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Make a Comment or Ask a Question
[contact-form-7]The post The Purpose of the CEO Perspectives Blog appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Pulmonary Arterial Hypertension is Associated with Elevated Levels of Galectin-3 and a Potential Therapeutic Target for GR-MD-02 appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>Galectin Therapeutics is collaborating with a number of academic groups to evaluate cardiovascular diseases that might be amenable to treatment with our galectin inhibitor drugs. In this article, I discuss the results obtained by the outstanding investigators at the Vascular Biology Center and the Department of Pharmacology and Toxicology at Augusta University (formerly known as Georgia Regents University), which were recently presented at the 2016 American Thoracic Society International Conference and highlighted in a recent press article.
What is pulmonary arterial hypertension (PAH)?
Pulmonary hypertension (PH) is high blood pressure in the main arteries that supply blood to the lungs, caused by the narrowing and constriction of blood vessels in the lungs themselves. This elevated blood pressure increases the work required of the heart’s right ventricle to pump blood into the lung, which eventually culminates in failure of the right ventricle, circulatory collapse and death. The causes of PH have been categorized into five groups. Group 1, also called pulmonary arterial hypertension (PAH), includes idiopathic and hereditary PAH, as well as multiple disorders that affect the small arterioles (blood vessels) in the lung. The remaining four groups are secondary to other disease including left heart failure (Group 2), chronic lung disease (Group 3), chronic pulmonary embolism (Group 4) or multifactorial mechanisms (Group 5).
The figure below on the left shows a normal pulmonary artery with the various layers of cells in the wall, including the external elastic lamina on the outside of the artery, the smooth muscle cell layer, the internal elastic lamina and the endothelial cell lining on the inside. As depicted in the figure below on the right, in PAH there is a marked increase in the thickness of the smooth muscle cell layer as well as fibrosis. This narrows the lumen of the artery and restricts blood flow.
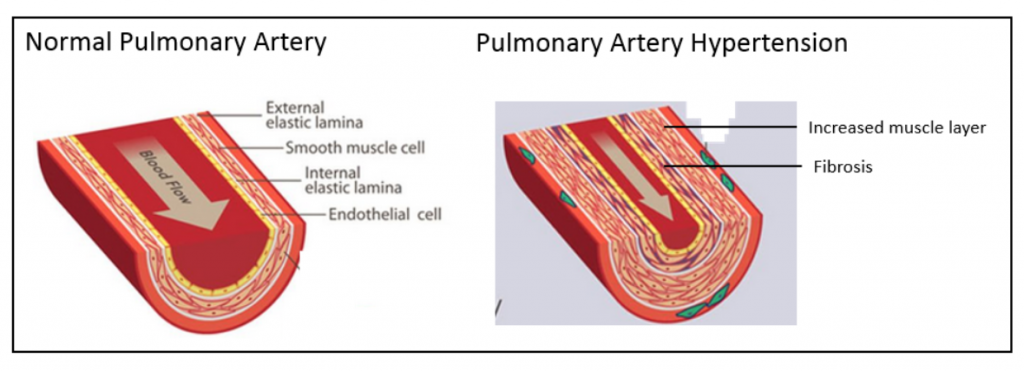
One of the more pressing needs is for new PH therapies in Group 1 disorders, particularly idiopathic PAH. Although there are a number of vasodilator drugs that are approved for the treatment of PAH, additional therapies are needed to address the structural narrowing of pulmonary arteries..
Galectin-3 protein is markedly elevated in human and experimental pulmonary arterial hypertension
The investigators at Augusta University experimented with three animal models of PAH using chemical treatments and/or low oxygen environments. These models are well established to cause narrowing of the pulmonary blood vessels leading to stress and failure of the right ventricle, meaning they mimic what happens in human PAH. The investigators found that the galectin-3 protein was markedly higher in the pulmonary arteries of diseased animals and the degree of increased blood pressure correlated with the level of galectin-3 elevation.
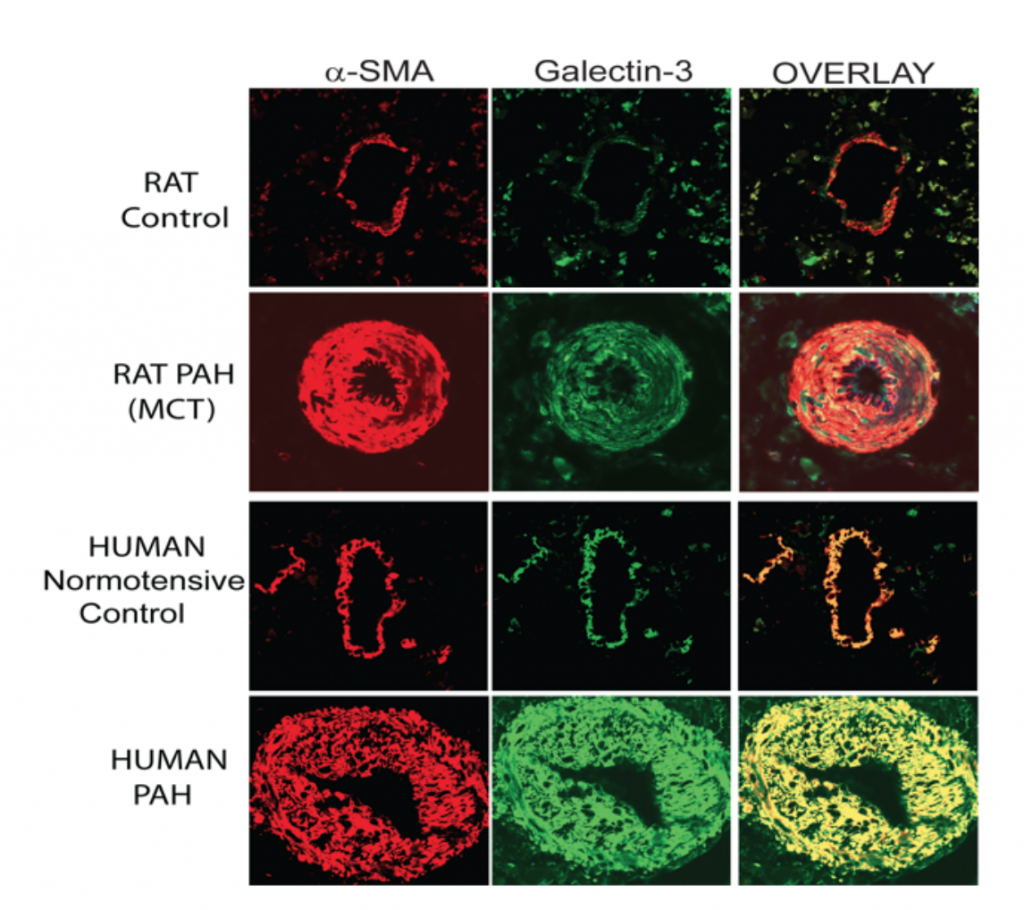
Since a picture is worth a thousand words, I have reproduced below images of the microscopic assessment of galectin-3 in pulmonary arteries in one rat model with monocrotaline treatment (MCT), as compared with the human disease.
The red stain is for the protein alpha smooth muscle actin (α-SMA), which stains smooth muscle cells in the artery, and the green stain is for the galectin-3 protein; the overlay combines the two images and shows that galectin-3 is predominantly in smooth muscle cells. In both the normal rat and the human pulmonary arteries, a thin layer of smooth muscle cells that contain galectin-3 is visible. In the rats and humans with PAH there is a marked increase in thickness of the muscle cells that surround the artery associated with a marked increase in galectin-3.
GR-MD-02 is effective in experimental pulmonary hypertension
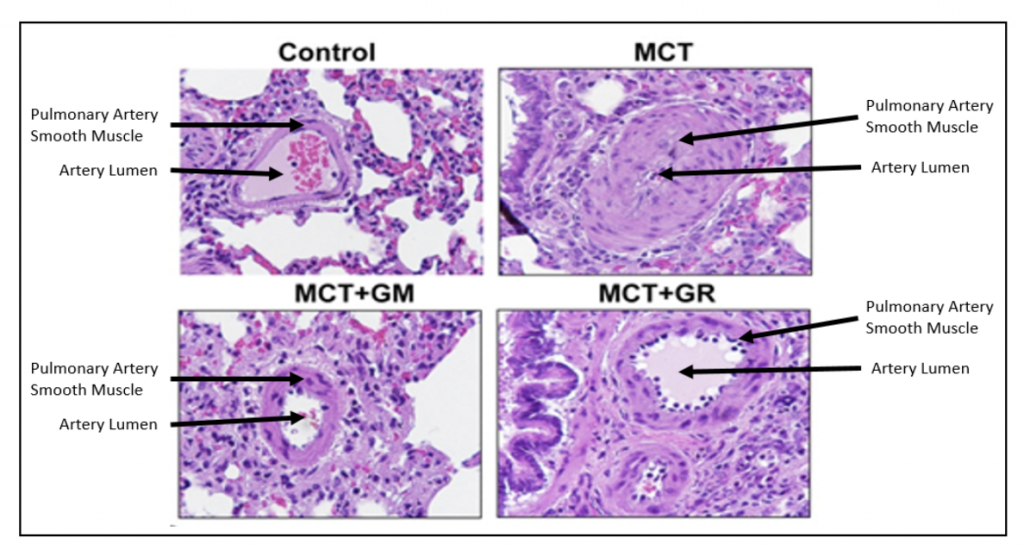
The investigators next tested whether our galectin-3 inhibitors were able to change the course of experimental PAH using the model in which the rats were given a single injection of MCT to induce pulmonary hypertension. Two of our galectin inhibitors were used as treatments: GR-MD-02, which is currently in clinical trials for NASH, psoriasis, and cancer, and GM-CT-01.
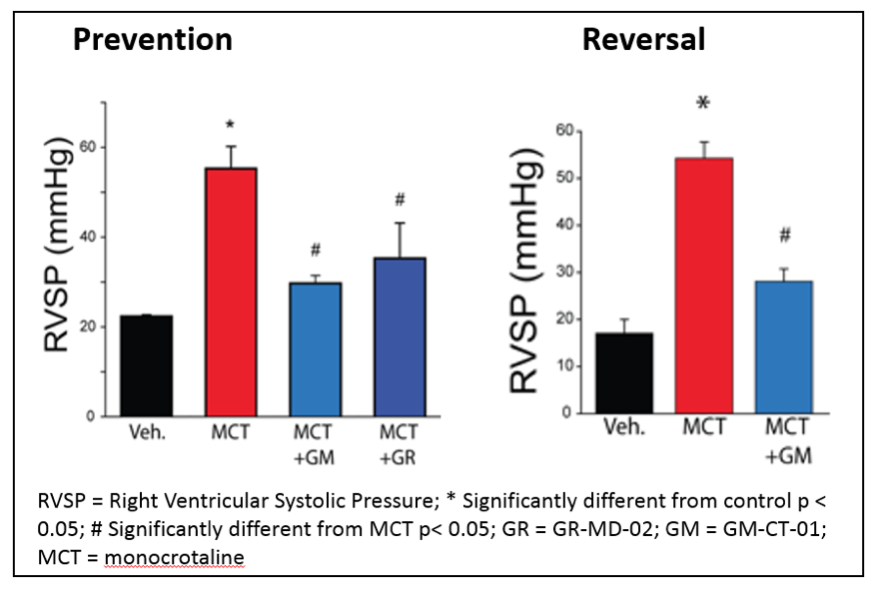
As shown in the figure below, the control (normal) rat pulmonary artery has a thin layer of smooth muscle cells surrounding it, whereas the animals treated with MCT have a markedly thickened smooth muscle layer that nearly blocks the lumen of the artery. Treatment of MCT animals with either GR-MD-02 or GM-CT-01 resulted in significantly fewer smooth muscle cells and a much larger artery lumen.
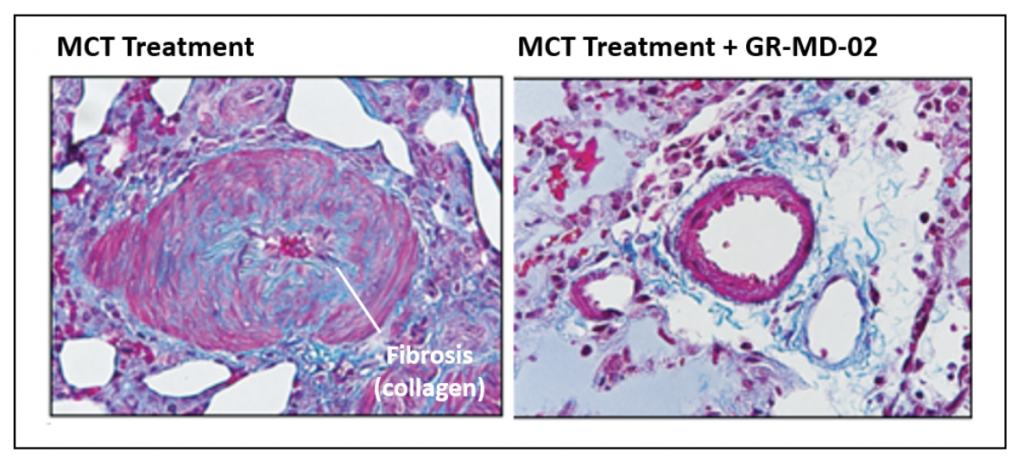
Below are images of pulmonary arteries stained to show fibrosis, mostly the protein collagen, which is identified by the light blue material. In the MCT treated animals, there is a significant amount of fibrosis associated with the smooth muscle cells in the pulmonary artery. This fibrosis is virtually eliminated in the artery wall following treatment with GR-MD-02, which provides a link to data showing improvement in fibrosis in other models of disease in liver, lung and kidney.
Treatment with either GR-MD-02 or GM-CT-01 also resulted in functional improvement in the PAH rats as indicated by reduced right ventricular systolic pressure (RVSP), as shown below. This improvement was seen whether the treatment was started immediately after the MCT injection (Prevention) or three weeks after the MCT injection (Reversal).
Prospects for human therapy
The results from these animal studies suggest that exploration of anti-galectin therapies, and specifically with GR-MD-02, might be a new therapeutic approach for these seriously ill patients. Currently there are approved therapies that help dilate constricted pulmonary arteries, but there are no therapies that effectively change the structure of the arteries or reverse the long-term course of the disease. A drug able to reverse the arterial smooth muscle and fibrosis findings in PAH might play an important role in treating these patients.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including whether GR-MD-02 and GM-CT-01 may be effective in the treatment of pulmonary arterial hypertension. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2015, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Make a Comment or Ask a Question
[contact-form-7]The post Pulmonary Arterial Hypertension is Associated with Elevated Levels of Galectin-3 and a Potential Therapeutic Target for GR-MD-02 appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post How Will We Interpret Results from the GR-MD- 02 Psoriasis Trial? appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>Last September, Galectin Therapeutics began a Phase 2a open-label clinical trial with GR-MD-02, our galectin-3 inhibitor drug, in patients with moderate-to-severe plaque psoriasis. We initiated this trial because a patient in our Phase 1 trial with GR-MD-02 in NASH had a remarkable and sustained remission of her psoriasis following treatment, as described in a previous CEO Perspective (here). Since then, I have been asked on numerous occasions how we will interpret the results of this trial.
What are the psoriasis trial endpoints?
Multiple drugs have been approved for the treatment of psoriasis, so there is an established FDA regulatory pathway to approval. It is well accepted that assessment of drug efficacy in psoriasis clinical trials is best performed using the Psoriasis Area and Severity Index, or PASI. PASI is used both to measure the severity of psoriasis as well as to measure the effect of treatment. While this index score is not generally used in the clinical practices of dermatology, it is universally used in clinical trials as an objective measurement of drug response.
PASI is an objective set of measurements that are conducted by a physician to assess the area of skin involvement and the severity of skin lesions in patients with psoriasis. The figure below, taken from a review article written by experts in the field, outlines the approach to the measurement (1).
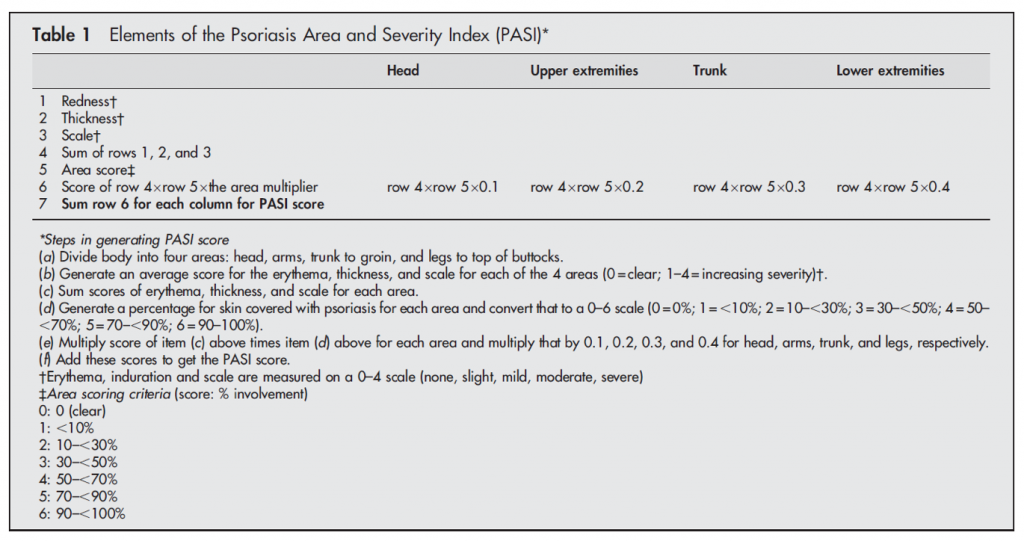
Therefore, the primary endpoint in our psoriasis trial will focus on improvement in PASI following treatment with GR-MD-02. We will evaluate the number of patients who have various percentage improvements in PASI. We will also record the durability of response to treatment for up to one year, should there be a beneficial effect. Finally, we are monitoring the incidence of adverse events and vital sign and laboratory abnormalities, if any, during study treatment.
What constitutes a meaningful effect?
Moderate psoriasis is generally defined as a PASI of ≥10. In our trial, as is typical for clinical trials in moderate-to-severe psoriasis, patients who enroll in the trial are required to have at least 10% of their body area affected by psoriasis and a PASI of ≥12, parameters which were agreed to with the FDA. Moreover, the patients will have the diagnosis of psoriasis confirmed by a skin biopsy. Therefore, patients in the trial will have significant disease and are similar to those who have been enrolled in the trials of approved drugs for psoriasis.
Once an individual has established psoriasis, it generally does not spontaneously dissipate or improve. As published in the medical literature, it has been suggested that clinically significant improvements in psoriasis are clearly obtained when PASI is lowered by 50% of baseline values, i.e., a PASI 50 response (2). However, the accepted clinical trial endpoint for drug registration is PASI 75, meaning a 75% reduction in psoriasis versus baseline. However, since this is an exploratory trial in which we do not have any information on optimal dosing, we will be looking for any objective response.
What will this trial tell us and what might be the next steps?
First and most important, our exploratory psoriasis clinical trial will either confirm the effect on psoriasis seen in the one Phase 1 patient, or show that her remission was unrelated to our drug. Should a significant proportion of patients in the trial respond to GR-MD-02 with an improvement in their psoriasis, such a finding would represent the first human disease response to GR-MD-02. That would be an important finding with potential implications for activity in our main therapeutic program for NASH, a disease where there is a higher incidence of psoriasis.
Second, this trial may indicate whether GR-MD-02 is a viable candidate to advance as a therapy for psoriasis. There are a number of biologic agents that have been approved in recent years for moderate-to-severe psoriasis, and GR-MD-02 would need to be competitive with those drugs. In my view, based on the efficacy of approved drugs, GR-MD-02 would need to demonstrate a PASI 75 in at least 50% of treated patients to consider full clinical development. If it does have such a degree of efficacy, it might be considered as a potential drug therapy on the basis of its lower cost to manufacture than existing drugs, a potentially better safety profile or the duration of effect after stopping treatment.
While each of these potential benefits would need to be studied in detail, the first step is to determine whether the drug has an effect on the disease, which is the primary goal of this study.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including whether GR-MD-02 may be effective in the treatment of psoriasis. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2015, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Reference List
1. Feldman SR, Krueger GG. Psoriasis assessment tools in clinical trials. Ann Rheum Dis 2005 Mar;64 Suppl 2:ii65-ii68.
2. Carlin CS, Feldman SR, Krueger JG, Menter A, Krueger GG. A 50% reduction in the Psoriasis Area and Severity Index (PASI 50) is a clinically significant endpoint in the assessment of psoriasis. J Am Acad Dermatol 2004 Jun;50(6):859-866.
Make a Comment or Ask a Question
[contact-form-7]The post How Will We Interpret Results from the GR-MD- 02 Psoriasis Trial? appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post The Patent Portfolio Strategy Behind GR-MD-02 appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>We often talk about how Galectin Therapeutics’ proprietary compound, GR-MD-02, is protected by a strong intellectual property portfolio. I thought it might be useful to drill a little more deeply into that statement.
Our patent portfolio is an important asset for Galectin Therapeutics, and it took hard and careful work to put it in place. Each patent/patent application is a strategic building block which reflects present and future business objectives and protects current core technology. The Galectin Therapeutics’ patent portfolio covers the products or composition of matter (i.e. active pharmaceutical ingredient or API), formulations of the products, use of the products and methods of manufacturing.
The most valuable patent in the Galectin Therapeutics’ portfolio is a composition of matter patent which covers the active pharmaceutical ingredient. Composition patents exclude others from making, using, selling or offering for sale the API.
Patenting complex carbohydrates can be more challenging than for a small molecule
Galectin Therapeutics’ proprietary compound, GR-MD-02, is derived from the modification of a natural pectin. Pectins are widely used in the food industry as a thickening agent for making things such as jellies and jams. There are a substantial number of patents on using pectin in pharmaceutical applications. Galectin Therapeutics had to show that GR-MD-02 was novel and non-obvious, in view of the prior pectin art.
GR-MD-02 is a complex carbohydrate molecule. A small molecule with a small molecular weight (e.g. 300 Da) in comparison can have a very specific structure and the U.S. Patent and Trademark Office (USPTO) can then compare the molecule to a chemical database to assess if the compound is different than publically available compounds.
GR-MD-02 on the other hand, is a mixture of complex carbohydrates having an average molecular weight between 20,000 to 70,000 Da. These are big molecules. This means that Galectin Therapeutics had to take a slightly different approach to characterizing the molecule in its patent applications. GR-MD-02 can be defined by its physical and chemical properties such as the average molecular weight, the carbohydrate composition, the ratio of critical carbohydrates from one to the other, and a two-dimensional NMR to establish a “fingerprint” of the molecule. One of the most critical carbohydrates in GR-MD-02 is galactose, and GR-MD-02 can be characterized by the ratio between galactose and arabinose. GR-MD-02 can be characterized by its degree of methoxylation — or the amount of methyl groups on the backbone of the molecule. Galectin Therapeutics was awarded a composition of matter patent in the U.S. on GR-MD-02 (U.S. patent 8,871,925 entitled “Compositions of Novel Carbohydrate Drug for Treatment of Human Diseases.” The U.S. patent issued on October 28, 2014 and will expire in 2032.
Protecting not just the molecule but also its manufacture and its use
Beyond the composition of matter patent for GR-MD-02 stands a portfolio of other patents that strengthen Galectin Therapeutics’ patent portfolio. Galectin Therapeutics holds a process patent that covers the manufacture GR-MD-02 from the pectin (U.S. patent No. 8,962,824). Presumably, a similar process has to be used to manufacture a drug similar to GR-MD-02, and having the process patented adds a second layer of protection to the compound as well.
Galectin Therapeutics also has 5 different patent families that cover the methods of use. These support the various indications for GR-MD-02 that Galectin Therapeutics plans to test. Method of use patents exclude others for using the API in specific indications. Galectin Therapeutics has a method of use U.S. patent for NASH (U.S. patent No. 8,658,787). Galectin Therapeutics has a method of use patent for kidney disease, specifically diabetic nephropathy as well as other similar types of glomerulopathy that patients develop, not necessarily related to diabetes (U.S. patent No. 8,828,971). We also have a method of use patent application allowed for pulmonary fibrosis, as well as method of use U.S. patents covering liver fibrosis, kidney fibrosis and heart fibrosis.
Patents pending
Galectin Therapeutics has more than 50 patent applications pending. There are a number of method of use patents pending, in both cancer immunotherapy and other disease states. The method of use patents and applications strengthen the patent portfolio and protect Galectin Therapeutics for future business objectives.
Foreign patents
Galectin Therapeutics has filed for patent protection in 10 foreign countries which are viewed are significant market for the API or the manufacture of the API (Australia, Brazil, Canada, China, Europe, Israel, Japan, Korea, Mexico and South Africa). The U.S. patent process generally moves more quickly than the rest of the world and issuance of patents in the U.S. can be helpful to expedite prosecution in foreign countries.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. These statements include those regarding the hope that Galectin Therapeutic’ s development program for GR-MD-02 will show that it can be both safe and effective when used in combination with other drugs for the treatment of patients with cancer. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Make a Comment or Ask a Question
[contact-form-7]The post The Patent Portfolio Strategy Behind GR-MD-02 appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post 2015 – 2016, Progress and Possibilities appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The many accomplishments at Galectin Therapeutics during 2015 ranged from incremental progress touching every aspect of our business to significant advancements with GR-MD-02. Importantly, this progress forms the basis for numerous milestones expected in the coming years including clinical progress, intellectual property fortification, further engagement with the investment community and ongoing outreach to educate our shareholders about our work to develop new therapies, the regulatory environment in which we operate and our target markets.
CEO Perspectives, Dr. Traber’s blog introduced last year, is designed to provide scientific and technical information largely regarding our work with GR-MD-02 in layman’s language. Much of our progress and accomplishments during 2015 were chronicled in the 14 blog postings, which can be found here.
We were delighted that in the final days of 2015 a U.S. District Court dismissed both the federal securities class-action lawsuit and the shareholder derivative actions lawsuit filed in the Summer of 2014 against Galectin and certain officers, directors and a shareholder, which had cast an inappropriate cloud over our many achievements in 2015. The Court entered final judgments of dismissals in both actions based on the Court’s finding that any further amendment of the complaints would be futile (i.e., dismissed with prejudice). Plaintiffs have the right to appeal the Court’s dismissals within 30 days. Based on the Federal Court’s rulings, Galectin is seeking dismissal of a duplicative shareholder derivative action in Nevada which was filed after the federal actions.
Our Clinical Programs
NASH with advanced liver fibrosis
Development of GR-MD-02 for the treatment of non-alcoholic steatohepatitis (NASH) with advanced fibrosis and cirrhosis continues to be the primary focus of our company. We completed a successful Phase 1 clinical trial and announced final data in January 2015. The Phase 1 trial demonstrated that GR-MD-02 is safe, with potential for therapeutic effect on fibrosis in NASH patients with advanced fibrosis. We found no serious adverse events and no treatment-emergent adverse events related to our drug. Furthermore, GR-MD-02 was found to be safe and well tolerated in each of the three dose-escalating cohorts of patients, who were suffering from NASH with advanced fibrosis.
This finding alone defines the study as a success, but we gained additional valuable information. We found that the FibroTest® score, a composite biomarker of five different blood tests that has been correlated with the extent of liver fibrosis, was significantly reduced by GR-MD-02 treatment in the third dosing cohort of 8 mg/kg. In addition, we found that some patients in this cohort also showed a decrease in liver stiffness, which has a direct correlation with fibrosis. We published a comprehensive piece on the results of the Phase 1 study in a CEO Perspectives blog post, which can be found here.
In addition to the phase 1 trial in NASH patients with advanced fibrosis, we reported the results of a drug-drug interaction study with GR-MD-02 and midazolam, a common sedative, which showed that in healthy volunteers there was no unfavorable interaction between the two compounds. Because many patients with chronic diseases are on multiple medications over long periods of time and may take other medications on an intermittent basis, this finding is important to the commercial potential of GR-MD-02 and to the patient population that is eligible to participate in our Phase 2 program. We published a CEO Perspectives piece on this topic, which is available here.
The information gleaned from the Phase 1 studies formed the basis for our Phase 2 program, which consists of two studies, one in NASH patients with advanced fibrosis and the other in NASH patients with cirrhosis. We submitted our protocol to the U.S. Food and Drug Administration (FDA) for the cirrhosis study in the first quarter, engaged our contract research organization and began screening patients at the end of June.
This study, the NASH-CX trial, is a multicenter, randomized, placebo-controlled, double-blind, parallel-group Phase 2 trial to evaluate the safety and efficacy of GR-MD-02 for the treatment of liver fibrosis and resultant portal hypertension (HVPG) in patients with NASH cirrhosis. A total of 156 patients at approximately 50 sites in the U.S. will be randomized to receive either 2 mg/kg of GR-MD-02, 8 mg/kg of GR-MD-02 or placebo, with 52 patients in each arm. The primary endpoint is a reduction in HVPG. Patients will receive a total of 26 infusions every other week for one year, at which time they will be evaluated for change in HVPG compared with placebo. HVPG will be correlated with secondary endpoints of fibrosis on liver biopsy as well as with measurement of liver stiffness via FibroScan® and assessment of liver metabolism (13C-methacetin breath test, Exalenz), which are non-invasive measures of the liver that may be used in future studies. More information can be found at www.clinicaltrials.gov and in a CEO Perspectives blog post, which can be found here.
We are pleased with the pace of the NASH-CX study and we remain on track to provide data readout in at the end of 2017, as we have previously indicated.
In September we initiated a 30-patient study with GR-MD-02 in NASH patients with advanced fibrosis, our NASH-FX study, with 15 patients receiving 8 mg/kg of GR-MD-02 and 15 patients receiving placebo every other week for 16 weeks. This study will evaluate the safety and efficacy of GR-MD-02 on liver fibrosis using multi-parametric magnetic resonance imaging (LiverMultiScan®, Perspectum Diagnostics) as the primary endpoint and liver stiffness as assessed by magnetic resonance-elastography and FibroScan as secondary endpoints. This study is also proceeding as planned, with top-line data expected around the end of the third quarter of 2016. We published a CEO Perspectives piece on this trial, which is available here.
Psoriasis
As we have previously reported, one of the patients participating in our Phase 1 NASH study was a long-term psoriasis sufferer, and this patient’s psoriasis cleared as the study progressed, and remained cleared for many months following the conclusion of the study. With an established theoretical pathway for how inhibition of galectin-3 might affect psoriasis, in September we began an open label 10-patient Phase 2a pilot study in patients with moderate-to-severe plaque psoriasis. We expect data readout from this study late in the third quarter of 2016. More information and background on this study can be found here.
Melanoma
We continued to support independent research with GR-MD-02 in combination with two commercial melanoma drugs, as preclinical research has shown our compound enhances the efficacy of immune checkpoint blockade therapies, or so-called checkpoint inhibitors, a new class of drugs. GR-MD-02 is progressing through a Phase 1b study in combination with Yervoy®, and a Phase 1b study in combination with Keytruda® was initiated in the fourth quarter of 2015 with enrollment to begin early in 2016. Preclinical work in mouse cancer models with GR-MD-02 added to checkpoint inhibitors shows a boost in anti-tumor immunity, a reduction in tumor size and increased survival. Both of these studies are being conducted at the Providence Cancer Center in Portland, Oregon. Galectin is providing GR-MD-02 to the investigators, who are funding the costs of these studies. We published a CEO Perspectives on these trials, which is available here.
In the trial combining Yervoy and GR-MD-02, two dosing cohorts have been completed and the third cohort delivering 4 mg/kg of GR-MD-02 is enrolling now. Of the seven patients that have received the combination therapy, there has been no dose limiting toxicity. Following completion of the 4 mg/kg dose cohort, a total of 10 patients will be dosed at 8 mg/kg. Immune markers as well as tumor response are being monitored in this study.
Significant Presentations and Publications
Galectin’s researchers presented at several important industry meetings during the year. Dr. Traber delivered an invited presentation of the company’s research with GR-MD-02 in NASH at the American Association for the Study of Liver Diseases (AASLD) Industry Colloquium in March. He participated in the session entitled “NASH: Clinical Endpoints and Drug Development” and discussed the role of galectin-3 in organ fibrosis generally and liver fibrosis in particular, and GR-MD-02 as a galectin-3 inhibitor. He reviewed the published preclinical data showing that GR-MD-02 is effective in reversing inflammation and fibrosis in a mouse model of NASH and also in reversing cirrhosis and improving portal hypertension in a rat model of cirrhosis. He also reviewed the company’s Phase 1 study results and its Phase 2 clinical program design. The abstract of Dr. Traber’s presentation can be found here.
In addition, preclinical research from a study led by Stefanie Linch, Ph.D. in the laboratory of tumor immunology expert William L. Redmond, Ph.D. of the Providence Cancer Center’s Earle A. Chiles Research Institute was presented in November at the Society for Immunotherapy of Cancer’s (SITC) 30th Anniversary Annual Meeting. The studies presented were conducted by the Institute in collaboration with Galectin Therapeutics. The poster presentation “Galectin-3 inhibition using novel inhibitor GR-MD-02 improves survival and immune function while reducing tumor vasculature” and an abstract was published in the Journal for ImmunoTherapy of Cancer. The poster presentation is available for review here.
Interviews with Dr. Traber appeared in a number of publications throughout 2015, including R&D Magazine in a piece entitled “Finding the Holy Grail Treatment for Fatty Livers,” available here, Obesity News Today published its Q&A article entitled, “Exclusive: Dr. Peter Traber Discusses Non-alcoholic Fatty Liver Disease”, available here, and MD Magazine, which conducted an online interview with Dr. Traber at the AASLD meeting. That interview can be found here.
Galectin management also participated in a number of investment conferences throughout the year, including programs for institutional investors, retail investors and family offices.
Foundational Support for our Business
During 2015 we considerably strengthened our intellectual property portfolio and received a U.S. patent Notice of Allowance for the use of pectin compounds to reduce fibrosis in multiple diseases. This patent is particularly important because it not only permits GR-MD-02 use for NASH with fibrosis, but it covers other compounds in our pipeline and a multitude of diseases with a fibrotic etiology. We also continued to build our international patent portfolio with patents issued or allowed in Israel and Australia.
We also made excellent progress with our Chemistry, Manufacturing and Controls (CMC), all of which are vital to the proper conduct of our clinical trials with GR-MD-02 and are essential components of the final application to the FDA for a drug’s approval. We discussed this progress during the year in the CEO Perspective post found here. We also reached a very significant milestone in our preclinical toxicology program, having completed chronic administration of GR-MD-02 in two animal species, allowing chronic administration in human subjects.
Lastly, we were very pleased to have completed a $9.8 million financing during the fourth quarter. This capital is expected to fund currently planned operations through the first quarter of 2017, and will be used mainly for clinical trial expenses and other research and development expenses, as well as for general corporate purposes.
Looking Ahead to 2016
We are looking forward to executing on several important milestones in 2016, with highlights including the following:
- We will continue enrollment in the NASH-CX study and work with our investigators and contract research organization to keep on our stated timelines for data readout in late 2017
- We will continue enrollment in the NASH-FX study, and continue to expect data readout around the end of the third quarter of 2016
- We will continue enrollment in the psoriasis Phase 2a study, with data readout also expected at the end of the third quarter of 2016
- While we do not control the rate of enrollment of the trial, we expect data from the Providence Cancer Center’s study with Yervoy in combination with GR-MD-02 in advanced metastatic melanoma by the end of 2016.
- We expect that Providence Cancer Center will be enrolling patients in the study with GR-MD-02 in combination with Keytruda during 2016.
We are fully aware that yesterday’s accomplishments set tomorrow’s expectations, and we look forward to creating shareholder value by executing on numerous milestones during 2016. We are grateful to our long-standing, loyal shareholders for their continued support and to the hard-working staff at Galectin Therapeutics who share a unifying commitment to addressing significant unmet clinical needs in NASH, as well as in oncology and psoriasis.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. These statements include those regarding the hope that Galectin Therapeutic’ s development program for GR-MD-02 will show that it can be both safe and effective when used in combination with other drugs for the treatment of patients with cancer. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements
Make a Comment or Ask a Question
[contact-form-7]The post 2015 – 2016, Progress and Possibilities appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Second Phase 2 clinical trial initiated for fatty liver disease with advanced fibrosis appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>As a company, we are focused on developing a therapy for patients with non-alcoholic steatohepatitis (NASH), or fatty liver disease, with advanced fibrosis (scarring) of the liver. Our drug GR-MD-02 has been shown to prevent and reverse fibrosis in preclinical animal models and there was evidence of a therapeutic effect in our Phase 1 trial (see previous CEO Perspectives). With the recent start of our second Phase 2 clinical trial (see September 16, 2015 press release), we now have studies underway that address two distinct NASH patient populations including those with cirrhosis, the most advanced stage of fibrosis, and those with advanced fibrosis without cirrhosis.
The newly announced NASH-FX clinical trial, discussed here, will attempt to show reversal of advanced fibrosis in a pre-cirrhotic stage. The goal is to reduce fibrosis to an early stage, slowing or preventing the patient from progressing to cirrhosis. Therefore, the NASH-FX trial addresses a very different patient population than the NASH-CX trial which treats NASH cirrhosis (see previous CEO Perspectives), which could lead to a separate indication.
The NASH-FX trial will treat NASH patients with advanced fibrosis with 8 mg/kg of GR-MD-02 or placebo for four months (see diagram below). The Principal Investigator, a prominent liver physician, and we believe that this is a sufficiently long period of time to show an effect on fibrosis given the results and treatment time of the Phase 1 trial. Additional trial details can be found at: https://clinicaltrials.gov/ct2/show/NCT02421094?term=GR-MD-02&rank=4
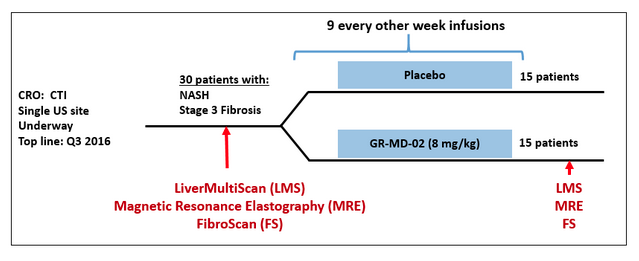
In the NASH-FX trial we will be using non-invasive imaging tests of the liver to assess fibrosis. The most definitive test for liver fibrosis involves microscopic evaluation of liver tissue obtained via a biopsy. The NASH-CX trial, for example, is using liver biopsy as one of the multiple evaluations of fibrosis in that trial. However, the short duration of the NASH-FX trial precludes repeated liver biopsies in patients because of safety concerns and potential discomfort related to the biopsies.
Liver biopsy is not a perfect test, as interpretation of liver biopsies is fraught with inconsistencies due to sampling error since the tissue piece obtained only represents approximately 1/50,000th of the liver. These inconsistencies along with the risk of the procedure itself have led investigators across the world to seek better, non-invasive approaches to evaluate liver fibrosis. One approach is to evaluate blood tests that may reflect the state of fibrosis in the liver, but this has not yet yielded results that are useful in clinical trials.
Better results have been obtained with methods to image the liver and assess physical characteristics of the liver tissue that is reflective of fibrosis. In the NASH-FX trial, we are using three of the most promising methods for directly assessing physical properties of the liver that correlate with fibrosis. The primary endpoint will be an assessment of fibrosis over the entire liver using multi-parametric magnetic resonance imaging (LiverMultiScan®), which is a validated and proprietary MRI protocol of Perspectum Diagnostics. Published information shows that this method is a sensitive approach to demonstrate differences in the degree of liver fibrosis (1). We chose this as the primary endpoint because data from Perspectum Diagnostics shows there are only small variations in repeated tests in the same individual.
Secondary endpoints will evaluate liver stiffness, which correlates to the degree of liver fibrosis, as assessed by magnetic resonance-elastography (MRE) and FibroScan® (FS). MRE provides a measure of liver stiffness over the entire imaged organ and has high diagnostic accuracy for detection of fibrosis in NASH, independent of BMI and degree of inflammation (2, 3). FS provides a measure of stiffness in a region of liver tissue about 100 times larger than a liver biopsy and also has good diagnostic accuracy for detecting liver fibrosis (4). These three tests will provide information on drug effect, as well as on the performance of the three tests relative to one another, which may indicate the best test(s) to utilize in future studies.
How will the results of the NASH-FX trial advance GR-MD-02 toward approval? With successful improvement in non-invasive fibrosis assessments, we will have proof-of-concept that treatment with GR-MD-02 over four months may be useful in this patient population. Because we are using actual physical liver measurements that correlate with fibrosis, positive study results will significantly improve the odds of success with future clinical trials and inform the dose and duration of treatment moving forward. While this trial alone will not be sufficient for drug approval for this indication, it will be critical for the design of pivotal trials for the purpose of marketing approval. We anticipate results for the NASH-FX trial will be available in the third quarter of 2016.
These “CEO Perspectives” will be a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “could,” “expect” and others. These statements include those regarding the hope that Galectin’s development program for GR-MD-02 will lead to the first therapy for the treatment of fatty liver disease with advanced fibrosis and cirrhosis. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements
Please look for future editions in which multiple aspects of our development programs for unmet medical needs will be addressed.
Reference List
1. Banerjee R, Pavlides M, Tunnicliffe EM, Piechnik SK, Sarania N, Philips R, et al. Multiparametric magnetic resonance for the non-invasive diagnosis of liver disease. J Hepatol 2014 Jan;60(1):69-77.
2. Singh S, Venkatesh SK, Loomba R, Wang Z, Sirlin C, Chen J, et al. Magnetic resonance elastography for staging liver fibrosis in non-alcoholic fatty liver disease: a diagnostic accuracy systematic review and individual participant data pooled analysis. Eur Radiol 2015 Aug 28.
3. Singh S, Venkatesh SK, Wang Z, Miller FH, Motosugi U, Low RN, et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: a systematic review and meta-analysis of individual participant data. Clin Gastroenterol Hepatol 2015 Mar;13(3):440-451.
4. Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le BB, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010 Feb;51(2):454-462.
Make a Comment or Ask a Question
[contact-form-7]The post Second Phase 2 clinical trial initiated for fatty liver disease with advanced fibrosis appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Lead product candidate GR-MD-02 shows no unfavorable drug-drug interactions appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>An important question in drug development, and the subject of U.S. Food and Drug Administration (FDA) regulations, is whether a drug candidate interacts with other medications the target patient population may also be taking. This is a critical issue, because many patients with chronic diseases are on multiple medications over long periods of time and may take other medications on an intermittent basis.
The basis of many drug-drug interactions is that the experimental new drug may use some of the same metabolic pathways that other drugs use. If this is the case, the new drug may inadvertently increase the levels of other drugs in the system, and thus alter their effect and increase their side effects. Prescribing physicians need to know about potential unfavorable interactions, if any, between an experimental new drug like GR-MD-02 and other drugs their patients may require.
In evaluating potential drug interactions, the first experiments with GR-MD-02 were done in test tubes and with cell cultures. Many metabolic pathways were evaluated in this way to determine whether there were potential interactions with GR-MD-02. Generally, the results of these experiments showed there was little to no risk of drug metabolism interactions with GR-MD-02, but the analysis of one particular metabolic enzyme, called CYP3A4, suggested there was small risk for interaction with other drugs. Therefore, the company agreed with the FDA to evaluate the possible interaction in humans of GR-MD-02 with a model drug for the CYP3A4 enzyme. The model drug used was midazolam, which is known as Versed® and is widely used for mild, conscious sedation.
We designed, conducted, completed, disclosed publicly (see our press release dated May 14, 2015) and reported to the FDA results of a Phase 1 study in normal healthy volunteer subjects. This study first tested drug levels of midazolam after a single intravenous (IV) dose, which served as a control. Midazolam was then administered again and drug levels were monitored following a single IV dose of GR-MD-02 (8 mg/kg) and following three weekly IV doses of GR-MD-02 (also 8 mg/kg). A total of 17 subjects completed the study, and all met the primary endpoint of no difference between midazolam levels when administered alone and in combination with single and multiple doses of GR-MD-02. Of note, 8 mg/kg of body weight of GR-MD-02 is the same dose we are testing in our current Phase 2 program in nonalcoholic steatohepatitis (NASH) patients with cirrhosis and with advanced fibrosis.
These Phase 1 results show that GR-MD-02 has no effect on the metabolism and serum levels of midazolam, and these results can be imputed to other drugs metabolized by CYP3A4 that are in common use. In fact, the CYP3A4 enzyme metabolizes about half of all the drugs currently on the market, according to published estimates. (Click here for more information on CYP3A4.) With the successful completion of this study, the company does not anticipate further drug-drug interaction studies will be required.
So, what does this science mean for the development of GR-MD-02? First, another 17 healthy individuals received up to three doses of GR-MD-02 without any significant adverse events, confirming the safety of the drug as seen in the first Phase 1 trial. Second, these findings allow patients on concomitant medications to be enrolled in our Phase 2 clinical trials with minimal concern for drug interactions, thus increasing the pool of potential patients that can be included in the trials. Finally, should GR-MD-02 receive marketing approval, patients and physicians will be less concerned our drug will interfere with other drugs they may be taking and we will not be faced with restrictive labeling regarding concomitant drug therapy. Therefore, successful completion of this drug interaction study in people checks another box in describing the underlying properties to support approval of GR-MD-02.
These “CEO Perspectives” will be a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “could,” “expect” and others. These statements include those regarding the hope that Galectin’s development program for GR-MD-02 will lead to the first therapy for the treatment of fatty liver disease with advanced fibrosis and cirrhosis. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements
Please look for future editions in which multiple aspects of our development programs for unmet medical needs will be addressed.
Make a Comment or Ask a Question
[contact-form-7]
The post Lead product candidate GR-MD-02 shows no unfavorable drug-drug interactions appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Successful Phase 1 Clinical Trial Supports Phase 2 Clinical Development Program appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>Galectin Therapeutics completed and reported on a successful Phase 1 clinical trial in NASH patients with advanced fibrosis. In a previous “CEO Perspective”, I described the general outline of our Clinical development program. We are using the galectin-3 inhibitor GR-MD-02 in a development program for potential treatment of patients with liver fibrosis, and specifically in those patients with NASH (non-alcoholic steatohepatitis) with advanced fibrosis or the most severe form of fibrosis, cirrhosis. The successful Phase 1 clinical trial was a critical step in this development program. What exactly did this trial show and why do we consider it successful?
The Phase 1 trial was the first time GR-MD-02 was administered to humans, but in addition it was designed to get the most information possible. First, unlike many phase 1 trials that include normal volunteers, the people in our trial had NASH with advanced fibrosis as determined from a liver biopsy—in other words one of the targeted groups for this therapy. Second, the trial was a placebo controlled and blinded trial meaning some patients got water instead of drug and neither patients nor medical workers knew if they were receiving the drug or water. The study included three sequential cohorts of patients, with each cohort receiving an escalated dose of GR-MD-02 (2, 4, and 8 mg/kg) administered by IV infusion over one hour. Finally, each patient received a total of four doses. More details on the protocol can be found at https://clinicaltrials.gov/ct2/show/NCT01899859?term=GR-MD-02&rank=2.
The most important aspect of a Phase 1 trial is an assessment of patient safety. In fact, that is the primary objective of Phase 1 trials. We found that there were no serious adverse events and no treatment emergent adverse events related to our drug GR-MD-02. Therapy emergent adverse events, all mild and transient, possibly related to study drug were reported in 4 subjects who received placebo and 2 subjects who received active drug. Thus, GR-MD-02 was found to be safe and well tolerated in this group of patients. This finding alone defines the study as a successful Phase 1 trial, but we gained a lot more information from the study.
Measures of blood levels of GR-MD-02 were done at multiple time points while the patients received the drug. Why is this important? From these blood levels we can calculate the total patient exposure to the drug to patients; in other words how high the blood levels are over a period of time. We can then use these exposure levels in humans to correlate with exposure levels in multiple studies which assessed the efficacy and safety of the drug in animal models. An analysis of this information showed us that the doses given to humans were in the ranges that were both safe and had an effect on NASH and fibrosis in animal studies. This is very valuable information for designing further studies of the drug.
Although the duration of this Phase 1 study was short, we additionally wanted to get an understanding of whether the drug was having any effect on the underlying disease in the treated patients. Liver biopsy assessment of fibrosis has historically been the gold standard for evaluating the effect of an anti-fibrotic drug, but liver biopsies cannot be safely repeated in a short clinical study because of the potential risks of taking a second biopsy too soon following the initial biopsy. Therefore, we evaluated a number of biomarkers (blood tests) that may reflect the state of fibrosis in the liver. There are currently no validated serum biomarkers for evaluation of potential therapeutic changes over time in NASH or fibrosis. Therefore, we evaluated a panel of serum tests to explore potential biomarkers for use in future studies. Most of the putative biomarkers showed high variability within the same individual in placebo and GR-MD-02 patients, rendering them not useful as reliable biomarkers.
The FibroTest® score, a composite of five different blood tests that has been correlated with the extent of liver fibrosis (1), was significantly reduced by GR-MD-02 treatment in cohort 3. The reduction in FibroTest Scores were almost entirely due to a reduction in alpha-2 macroglobulin levels, one of the blood tests that make up the FibroTest, as shown in the figure below. This figure shows that the first two dosage groups of GR-MD-02 (cohort 1 and 2) did not have a significant difference in the change of alpha-2 macroglobulin as compared to placebo-treated patients. Note that some have incorrectly stated that cohort 2 was worse than placebo (eg, it went up) when in fact the difference was not significant and, thus due to randomness of blood values; in other words there was no difference between placebo or the first two cohorts. However, if one looks at cohort 3 (the highest dose used), there was a statistically significant reduction at three different time points for the blood sampling; just before the second dose, 3 days following the fourth dose, and 14 days following the fourth dose. Additional blood samples were taken in cohort 3 to evaluate biomarkers more effectively and to correlate with both cohort 1 and 2. For those readers not used to evaluating statistics, a p value of less than 0.001 means that there is less than a one in a thousand chance that the difference from placebo is due to chance.
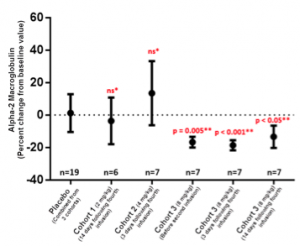
The reduction in alpha-2 macroglobulin (A2MG) is of interest because this protein is a relevant marker for fibrosis. In fact, in a recent human study, A2MG was found by the authors to be “the most important serum biomarker for liver fibrosis” among the multiple markers they evaluted (2). It is known to inhibit multiple proteases, including collagenase, which has been hypothesized to promote fibrosis. It is expressed in liver cells, a subset of macrophages, and stellate cells, where its expression is increased as they are converted to collagen producing cells. Finally, clinical studies show that alpha-2 macroglobulin increases with increasing liver fibrosis as assessed by liver biopsy. The reduction seen in alpha-2 macroglobulin suggests there may be changes in the fibrogenic process that might lead to an improvement in fibrosis with longer term therapy.
It was decided before cohort 3 started to add a physical evaluation of the liver, FibroScan® to assess directly for liver fibrosis in patients who were being treated at facilities who had Fibroscan® equipment. FibroScan® uses an electromechanical vibrator and pulse-echo ultrasound to evaluate the elastic shear wave in liver tissue. The volume of liver tissue assessed is ~100-times greater than volume assessed by liver biopsy. The stiffness of the liver is recorded as a pressure measurement of kPa (kilopascals) and the stiffness of the liver has been shown to correlate with the degree of liver fibrosis as assessed by liver biopsy. For this reason, FibroScan® represents a promising non-invasive, out-patient method for measuring changes in liver fibrosis over time, including patients with NASH (3).
Technically adequate FibroScan® evaluations were obtained scans at baseline, Day 38 and Day 63 in 5 patients administered GR-MD-02 and 3 patients administered placebo. As shown in the figure below, there was essentially no change in the liver stiffness for the three placebo patients, with all FibroScan scores within 20% of baseline. In contrast, 3 of the 5 patients treated with GR-MD-02 had a reduction in liver stiffness below 20% of the baseline value, with two patients having a reduction of 50% from baseline. While these numbers of patients are small, this suggests that liver stiffness may decrease in NASH patients with advanced fibrosis who received GR-MD-02.
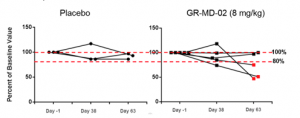
The results of this Phase 1 study was successful in providing information on the design of a Phase 2 clinical trial to demonstrate efficacy. First, and most importantly, GR-MD-02 was safe and well tolerated at single and multiple doses of 2, 4, and 8mg/kg. Pharmacokinetics (blood levels) revealed drug exposure in humans at the 8 mg/kg dose that was equivalent to the upper range of the targeted therapeutic dose determined from effective doses in NASH animal models, thus providing support for the proposed Phase 2 dosing regimen. Additionally, there was evidence of an effect on a relevant disease marker, with a dose dependent reduction in FibroTest® scores due to a reduction in alpha-2 macroglobulin levels. And finally, there was a trend that suggested liver stiffness, which may be related to a reduction in fibrotic tissue, is reduced by GR-MD-02. While health related outcomes or liver biopsies was not appropriate to be evaluated in this short-term treatment Phase 1 trial, it does appear that there was an effect of GR-MD-02 on the disease process.
On the basis of this Phase 1 study, Galectin has initiated two phase 2 clinical trials to evaluate the possible reversal of liver fibrosis in NASH, the most common form of liver disease. The NASH-CX trial will focus on reversal of fibrosis in the most severe form of fibrosis called cirrhosis and the NASH-FX trial will attempt to show reversal of advanced fibrosis in a pre-cirrhotic stage.
More information on Galectin’s clinical trials can be found at clinicaltrials.gov
- NASH-CX: https://clinicaltrials.gov/ct2/show/NCT02462967?term=GR-MD-02&rank=3
- NASH-FX: https://clinicaltrials.gov/ct2/show/NCT02421094?term=GR-MD-02&rank=4
These “CEO Perspectives” will be a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may”, “could”, “expect” and others. These statements include those regarding the hope that Galectin’s development program for GR-MD-02 will lead to the first therapy for the treatment of fatty liver disease with advanced fibrosis and cirrhosis. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements
Please look for future editions in which multiple aspects of our development programs for unmet medical needs will be addressed.
Reference List
- Poynard T, Morra R, Halfon P, Castera L, Ratziu V, Imbert-Bismut F, et al. Meta-analyses of FibroTest diagnostic value in chronic liver disease. BMC Gastroenterol 2007;7:40.
- Atanasova E, Martinova F, Jelev D, Antonov K, de Mey C, Mateva L, et al. Alpha-2 macroglobulin is the simplest serum biomarker for liver fibrosis and fibrogenesis in chronic hepatitis C. MedInform 2015;1(2):153-164.
- Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le BB, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010 Feb;51(2):454-462.
Make a Comment or Ask a Question
[contact-form-7]
The post Successful Phase 1 Clinical Trial Supports Phase 2 Clinical Development Program appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Welcome to CEO Perspectives appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>During the normal course of conducting business in a public company like Galectin Therapeutics, as the CEO and Chief Medical Officer of the company, I have conversations and interactions with many different firms and individuals on a daily basis. I am frequently discussing and fielding questions on science, our drug development programs, and many other topics from investors, analysts, scientists, physicians and others. At all times, the factual information that is discussed during these conversations has been previously publically disclosed and is available in our filings with the U.S. Securities and Exchange Commission. Everyone has access to all the information that I, or any other employee of the Company, discloses to individuals outside of the company.
In a highly technical area such as drug development, our investors and other interested members of the public have a great thirst for information and explanation of our programs I would like to help investors and members of the public understand our programs and answer questions that I routinely hear using a new communication medium that we designed, called “CEO Perspectives”. In this series of “CEO Perspectives” I will address key areas of activities for Galectin informed by questions that I receive from various constituents. It is my hope that these articles will help collate and clarify the already publically available information. Following each “CEO Perspective” there will be a comment section. Please use this to communicate your thoughts and questions. I hope you enjoy this new communication series and find it useful.
These “CEO Perspectives” will be a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may”, “could”, “expect” and others. These statements include those regarding the hope that Galectin’s development program for GR-MD-02 will lead to the first therapy for the treatment of fatty liver disease with advanced fibrosis and cirrhosis. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements
Please look for future editions in which multiple aspects of our development programs for unmet medical needs will be addressed.
Make a Comment or Ask a Question
[contact-form-7]The post Welcome to CEO Perspectives appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>