The post Results from the NASH-FX Study Underscore the Importance of Completing the NASH-CX Clinical Trial for Patients with NASH Cirrhosis appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The NASH-FX study was designed as a pilot study at a single site, involving only four months of treatment with GR-MD-02. This pilot study evolved from the results of a Phase 1 study in NASH patients with advanced fibrosis that had suggested FibroScan® measurements improved in three patients with just four doses of the study drug. The full report of this Phase 1 study will be published in the next few weeks in the peer-reviewed scientific journal, Alimentary Pharmacology and Therapeutics.
While most experts feel that liver fibrosis trials should have treatment phases for at least a year in duration, the results from the earlier Phase 1 NASH study provided a rationale for studying a larger group of patients with shorter therapy and exploring non-invasive technologies for assessing liver disease and fibrosis with a goal of using these technologies in later trials.
I want to give you a few details on the design of the NASH-FX study. The primary endpoint of NASH-FX was the LiverMultiScan (LMS), an approved magnetic resonance imaging test developed by Perspectum Diagnostics to assist in the diagnosis of liver disease. In NASH, the LMS is reported to measure the amount of fibrosis and inflammation. The LMS has low variability between scans in the same individual, and we used this low variability to calculate the number of patients we would need for the study to show a statistically significance difference between treatment groups with a power of 80%. In other words, the study was designed to have an 80% chance of showing a statistically significant difference in a 30 patient study, with 15 placebo and 15 GR-MD-02 patients. The power of the actual study, calculated after completion, was almost exactly an 80% chance to show an approximately 10% difference in LMS between placebo and treated groups. Therefore, the study design was adequate for the primary endpoint.
In contrast to LMS, the study was not powered for the secondary endpoints of liver stiffness, FibroScan and magnetic resonance elastography (MRE). The study would have required between 3 to 5 times as many patients to have an adequate power to show a difference with these tests. This is because the variability of these tests for repeated measurements is considerably greater than LMS.
However, we did not know before we conducted the study, nor did anyone else know, whether LMS correlated with the liver stiffness measurements of FibroScan and MRE. The NASH-FX study showed that there was poor correlation. Therefore, one cannot conclude that because there was no difference in LMS, that there would not be a difference in stiffness measurements, which have been shown in liver biopsy studies to correlate with fibrosis.
Although there was no apparent improvement in the three non-invasive tests for assessment of liver fibrosis in the four-month NASH-FX study, Dr. Stephen Harrison, a leading investigator in NASH and liver disease and the principal investigator of the NASH-FX study has pointed out that the inhibition of galectin-3 with GR-MD-02 remains promising for the treatment of NASH fibrosis. Dr. Harrison was especially encouraged that GR-MD-02 has demonstrated an improved clinical effect in moderate-to-severe psoriasis, suggesting the compound has activity in a human disease that can occur in association with NASH.
It remains critical that we complete the longer therapy NASH-CX clinical trial that has a much larger group of patients with NASH cirrhosis.
The NASH-CX trial is a one-year of treatment, multi-center trial in patients with NASH cirrhosis that is being conducted at 36 outstanding liver centers in the United States. The endpoints of the NASH-CX trial include invasive tests that are well-validated measures of liver disease severity. The primary endpoint is the change from baseline in the hepatic venous pressure gradient (HVPG), which measures the blood pressure in the liver and is well correlated with the clinical outcomes of patients.
Liver biopsy is an important secondary endpoint in the NASH-CX trial, which evaluates the stage of liver fibrosis and the amount of collagen, the primary component of fibrotic tissue. Finally, there are also non-invasive tests as secondary endpoints, including FibroScan and the 13C-methacetin breath test, which measures the metabolic function of the liver. These are important to correlate with the invasive tests because they may be useful in future trials and in management of patients.
I am pleased to report additional information on the status of this most important clinical trial as of October 10, 2016:
- The NASH-CX trial completed enrollment one month early with 162 total patients, exceeding the target of 156. This keeps us well on track for reporting of top-line results in December of 2017.
- The 162 patients were enrolled at 36 sites in the United States following the screening of 290 patients to obtain a population with well-compensated NASH cirrhosis (Child-Pugh-Turcotte Class A) with elevated portal pressure (HVPG ≥ 6 mmHg).
- In determining the number of patients to meet statistical requirements, we planned for the possibility that as many as 25% of the patients may drop out of the study during the treatment phase. However, we are pleased that only five patients of the 162 enrolled have dropped out of the study thus far. This low attrition rate highlights the importance, urgency, and need for patients suffering from NASH-cirrhosis to find an effective medical treatment.
- The low drop out trend also suggests that we will have a robust number of patients completing treatment for evaluation at the end of the trial. The trial was designed to have an 80% chance of demonstrating a 2 mmHg reduction in HVPG (i.e. 80% power) with 117 patients evaluated. Any number of patients above 117 will simply enhance the power of the study.
- At this point, 4 patients have completed the entire protocol and 70 patients have already completed six months of dosing.
- A total of 2,000 drug infusions (including placebo) have been given in this trial, representing 48% of the total number of infusions in the entire study. So we are quite pleased that this study is well along in its development.
The safety and tolerance of GR-MD-02 in all of the trials is most encouraging and supports our commitment to pursue the lead indication of NASH cirrhosis. In the NASH-FX study, GR-MD-02 was found to be safe and well-tolerated among the patient population with no serious adverse events related to the study medication. Over all of the clinical trials, including the patients in the NASH-CX trial, more than 1600 doses of GR-MD-02 have been administered without serious adverse effects related to the drug. This highlights the superior safety profile of the therapy in a patient population with advanced-stage disease, which is buttressed by the biological activity demonstrated in patients with moderate to severe plaque psoriasis.
Dr. Harrison, one of the two co-lead investigators in the NASH-CX trial, stated his belief that the inhibition of galectin-3 with GR-MD-02 remains a promising treatment for NASH fibrosis and that it is important to complete the NASH-CX trial.
Dr. Naga Chalasani, the other co-lead principal investigator of the NASH-CX trial, provided his assessment, stating:
“The results from the NASH-FX trial do not diminish the significance of the NASH-CX trial. Along with the safety and tolerability profile observed in the NASH-FX trial, the different patient population, much larger enrollment, rigorous study design and longer duration of therapy offer compelling rationale to complete the NASH-CX trial.”
As a company, Galectin Therapeutics’ attention has always been focused on completing the NASH-CX clinical trial and reporting results in a timely fashion.
With an outstanding safety profile, inhibition of galectin-3 with GR-MD-02 remains a potential treatment of NASH cirrhosis. Additionally, the longer therapy for one year, and endpoints that may serve as a surrogate for outcomes for registration trials in this patient population, provides us encouragement about our continuation of NASH-CX clinical trial.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including whether GR-MD-02 may be effective in the treatment of NASH. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2015, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Make a Comment or Ask a Question
[contact-form-7]The post Results from the NASH-FX Study Underscore the Importance of Completing the NASH-CX Clinical Trial for Patients with NASH Cirrhosis appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post How Will We Interpret Results from the GR-MD- 02 Psoriasis Trial? appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>Last September, Galectin Therapeutics began a Phase 2a open-label clinical trial with GR-MD-02, our galectin-3 inhibitor drug, in patients with moderate-to-severe plaque psoriasis. We initiated this trial because a patient in our Phase 1 trial with GR-MD-02 in NASH had a remarkable and sustained remission of her psoriasis following treatment, as described in a previous CEO Perspective (here). Since then, I have been asked on numerous occasions how we will interpret the results of this trial.
What are the psoriasis trial endpoints?
Multiple drugs have been approved for the treatment of psoriasis, so there is an established FDA regulatory pathway to approval. It is well accepted that assessment of drug efficacy in psoriasis clinical trials is best performed using the Psoriasis Area and Severity Index, or PASI. PASI is used both to measure the severity of psoriasis as well as to measure the effect of treatment. While this index score is not generally used in the clinical practices of dermatology, it is universally used in clinical trials as an objective measurement of drug response.
PASI is an objective set of measurements that are conducted by a physician to assess the area of skin involvement and the severity of skin lesions in patients with psoriasis. The figure below, taken from a review article written by experts in the field, outlines the approach to the measurement (1).
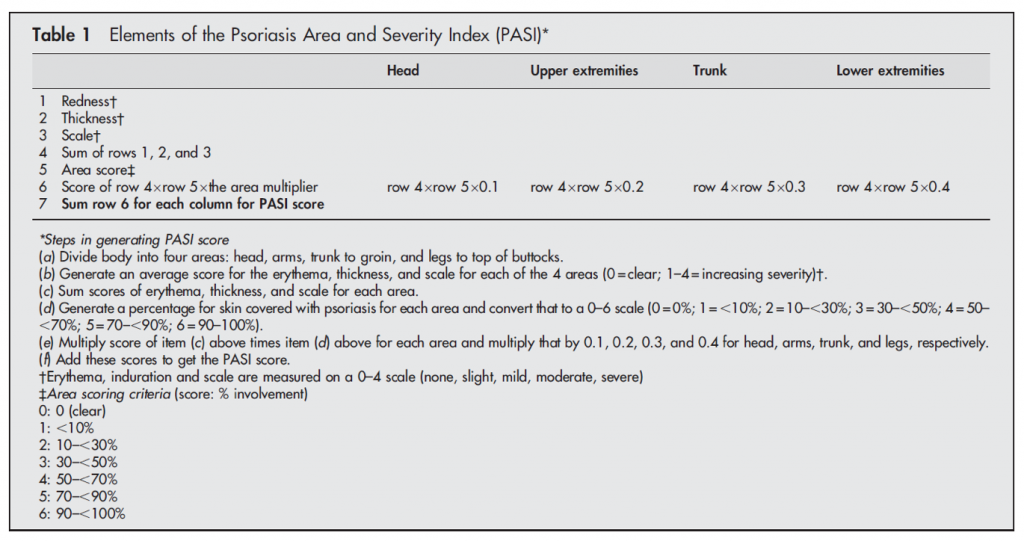
Therefore, the primary endpoint in our psoriasis trial will focus on improvement in PASI following treatment with GR-MD-02. We will evaluate the number of patients who have various percentage improvements in PASI. We will also record the durability of response to treatment for up to one year, should there be a beneficial effect. Finally, we are monitoring the incidence of adverse events and vital sign and laboratory abnormalities, if any, during study treatment.
What constitutes a meaningful effect?
Moderate psoriasis is generally defined as a PASI of ≥10. In our trial, as is typical for clinical trials in moderate-to-severe psoriasis, patients who enroll in the trial are required to have at least 10% of their body area affected by psoriasis and a PASI of ≥12, parameters which were agreed to with the FDA. Moreover, the patients will have the diagnosis of psoriasis confirmed by a skin biopsy. Therefore, patients in the trial will have significant disease and are similar to those who have been enrolled in the trials of approved drugs for psoriasis.
Once an individual has established psoriasis, it generally does not spontaneously dissipate or improve. As published in the medical literature, it has been suggested that clinically significant improvements in psoriasis are clearly obtained when PASI is lowered by 50% of baseline values, i.e., a PASI 50 response (2). However, the accepted clinical trial endpoint for drug registration is PASI 75, meaning a 75% reduction in psoriasis versus baseline. However, since this is an exploratory trial in which we do not have any information on optimal dosing, we will be looking for any objective response.
What will this trial tell us and what might be the next steps?
First and most important, our exploratory psoriasis clinical trial will either confirm the effect on psoriasis seen in the one Phase 1 patient, or show that her remission was unrelated to our drug. Should a significant proportion of patients in the trial respond to GR-MD-02 with an improvement in their psoriasis, such a finding would represent the first human disease response to GR-MD-02. That would be an important finding with potential implications for activity in our main therapeutic program for NASH, a disease where there is a higher incidence of psoriasis.
Second, this trial may indicate whether GR-MD-02 is a viable candidate to advance as a therapy for psoriasis. There are a number of biologic agents that have been approved in recent years for moderate-to-severe psoriasis, and GR-MD-02 would need to be competitive with those drugs. In my view, based on the efficacy of approved drugs, GR-MD-02 would need to demonstrate a PASI 75 in at least 50% of treated patients to consider full clinical development. If it does have such a degree of efficacy, it might be considered as a potential drug therapy on the basis of its lower cost to manufacture than existing drugs, a potentially better safety profile or the duration of effect after stopping treatment.
While each of these potential benefits would need to be studied in detail, the first step is to determine whether the drug has an effect on the disease, which is the primary goal of this study.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including whether GR-MD-02 may be effective in the treatment of psoriasis. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2015, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Reference List
1. Feldman SR, Krueger GG. Psoriasis assessment tools in clinical trials. Ann Rheum Dis 2005 Mar;64 Suppl 2:ii65-ii68.
2. Carlin CS, Feldman SR, Krueger JG, Menter A, Krueger GG. A 50% reduction in the Psoriasis Area and Severity Index (PASI 50) is a clinically significant endpoint in the assessment of psoriasis. J Am Acad Dermatol 2004 Jun;50(6):859-866.
Make a Comment or Ask a Question
[contact-form-7]The post How Will We Interpret Results from the GR-MD- 02 Psoriasis Trial? appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Successful Phase 1 Clinical Trial Supports Phase 2 Clinical Development Program appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>Galectin Therapeutics completed and reported on a successful Phase 1 clinical trial in NASH patients with advanced fibrosis. In a previous “CEO Perspective”, I described the general outline of our Clinical development program. We are using the galectin-3 inhibitor GR-MD-02 in a development program for potential treatment of patients with liver fibrosis, and specifically in those patients with NASH (non-alcoholic steatohepatitis) with advanced fibrosis or the most severe form of fibrosis, cirrhosis. The successful Phase 1 clinical trial was a critical step in this development program. What exactly did this trial show and why do we consider it successful?
The Phase 1 trial was the first time GR-MD-02 was administered to humans, but in addition it was designed to get the most information possible. First, unlike many phase 1 trials that include normal volunteers, the people in our trial had NASH with advanced fibrosis as determined from a liver biopsy—in other words one of the targeted groups for this therapy. Second, the trial was a placebo controlled and blinded trial meaning some patients got water instead of drug and neither patients nor medical workers knew if they were receiving the drug or water. The study included three sequential cohorts of patients, with each cohort receiving an escalated dose of GR-MD-02 (2, 4, and 8 mg/kg) administered by IV infusion over one hour. Finally, each patient received a total of four doses. More details on the protocol can be found at https://clinicaltrials.gov/ct2/show/NCT01899859?term=GR-MD-02&rank=2.
The most important aspect of a Phase 1 trial is an assessment of patient safety. In fact, that is the primary objective of Phase 1 trials. We found that there were no serious adverse events and no treatment emergent adverse events related to our drug GR-MD-02. Therapy emergent adverse events, all mild and transient, possibly related to study drug were reported in 4 subjects who received placebo and 2 subjects who received active drug. Thus, GR-MD-02 was found to be safe and well tolerated in this group of patients. This finding alone defines the study as a successful Phase 1 trial, but we gained a lot more information from the study.
Measures of blood levels of GR-MD-02 were done at multiple time points while the patients received the drug. Why is this important? From these blood levels we can calculate the total patient exposure to the drug to patients; in other words how high the blood levels are over a period of time. We can then use these exposure levels in humans to correlate with exposure levels in multiple studies which assessed the efficacy and safety of the drug in animal models. An analysis of this information showed us that the doses given to humans were in the ranges that were both safe and had an effect on NASH and fibrosis in animal studies. This is very valuable information for designing further studies of the drug.
Although the duration of this Phase 1 study was short, we additionally wanted to get an understanding of whether the drug was having any effect on the underlying disease in the treated patients. Liver biopsy assessment of fibrosis has historically been the gold standard for evaluating the effect of an anti-fibrotic drug, but liver biopsies cannot be safely repeated in a short clinical study because of the potential risks of taking a second biopsy too soon following the initial biopsy. Therefore, we evaluated a number of biomarkers (blood tests) that may reflect the state of fibrosis in the liver. There are currently no validated serum biomarkers for evaluation of potential therapeutic changes over time in NASH or fibrosis. Therefore, we evaluated a panel of serum tests to explore potential biomarkers for use in future studies. Most of the putative biomarkers showed high variability within the same individual in placebo and GR-MD-02 patients, rendering them not useful as reliable biomarkers.
The FibroTest® score, a composite of five different blood tests that has been correlated with the extent of liver fibrosis (1), was significantly reduced by GR-MD-02 treatment in cohort 3. The reduction in FibroTest Scores were almost entirely due to a reduction in alpha-2 macroglobulin levels, one of the blood tests that make up the FibroTest, as shown in the figure below. This figure shows that the first two dosage groups of GR-MD-02 (cohort 1 and 2) did not have a significant difference in the change of alpha-2 macroglobulin as compared to placebo-treated patients. Note that some have incorrectly stated that cohort 2 was worse than placebo (eg, it went up) when in fact the difference was not significant and, thus due to randomness of blood values; in other words there was no difference between placebo or the first two cohorts. However, if one looks at cohort 3 (the highest dose used), there was a statistically significant reduction at three different time points for the blood sampling; just before the second dose, 3 days following the fourth dose, and 14 days following the fourth dose. Additional blood samples were taken in cohort 3 to evaluate biomarkers more effectively and to correlate with both cohort 1 and 2. For those readers not used to evaluating statistics, a p value of less than 0.001 means that there is less than a one in a thousand chance that the difference from placebo is due to chance.
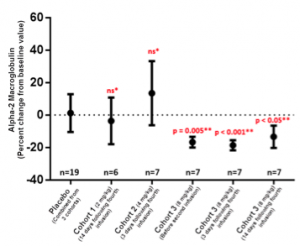
The reduction in alpha-2 macroglobulin (A2MG) is of interest because this protein is a relevant marker for fibrosis. In fact, in a recent human study, A2MG was found by the authors to be “the most important serum biomarker for liver fibrosis” among the multiple markers they evaluted (2). It is known to inhibit multiple proteases, including collagenase, which has been hypothesized to promote fibrosis. It is expressed in liver cells, a subset of macrophages, and stellate cells, where its expression is increased as they are converted to collagen producing cells. Finally, clinical studies show that alpha-2 macroglobulin increases with increasing liver fibrosis as assessed by liver biopsy. The reduction seen in alpha-2 macroglobulin suggests there may be changes in the fibrogenic process that might lead to an improvement in fibrosis with longer term therapy.
It was decided before cohort 3 started to add a physical evaluation of the liver, FibroScan® to assess directly for liver fibrosis in patients who were being treated at facilities who had Fibroscan® equipment. FibroScan® uses an electromechanical vibrator and pulse-echo ultrasound to evaluate the elastic shear wave in liver tissue. The volume of liver tissue assessed is ~100-times greater than volume assessed by liver biopsy. The stiffness of the liver is recorded as a pressure measurement of kPa (kilopascals) and the stiffness of the liver has been shown to correlate with the degree of liver fibrosis as assessed by liver biopsy. For this reason, FibroScan® represents a promising non-invasive, out-patient method for measuring changes in liver fibrosis over time, including patients with NASH (3).
Technically adequate FibroScan® evaluations were obtained scans at baseline, Day 38 and Day 63 in 5 patients administered GR-MD-02 and 3 patients administered placebo. As shown in the figure below, there was essentially no change in the liver stiffness for the three placebo patients, with all FibroScan scores within 20% of baseline. In contrast, 3 of the 5 patients treated with GR-MD-02 had a reduction in liver stiffness below 20% of the baseline value, with two patients having a reduction of 50% from baseline. While these numbers of patients are small, this suggests that liver stiffness may decrease in NASH patients with advanced fibrosis who received GR-MD-02.
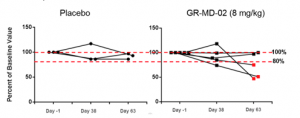
The results of this Phase 1 study was successful in providing information on the design of a Phase 2 clinical trial to demonstrate efficacy. First, and most importantly, GR-MD-02 was safe and well tolerated at single and multiple doses of 2, 4, and 8mg/kg. Pharmacokinetics (blood levels) revealed drug exposure in humans at the 8 mg/kg dose that was equivalent to the upper range of the targeted therapeutic dose determined from effective doses in NASH animal models, thus providing support for the proposed Phase 2 dosing regimen. Additionally, there was evidence of an effect on a relevant disease marker, with a dose dependent reduction in FibroTest® scores due to a reduction in alpha-2 macroglobulin levels. And finally, there was a trend that suggested liver stiffness, which may be related to a reduction in fibrotic tissue, is reduced by GR-MD-02. While health related outcomes or liver biopsies was not appropriate to be evaluated in this short-term treatment Phase 1 trial, it does appear that there was an effect of GR-MD-02 on the disease process.
On the basis of this Phase 1 study, Galectin has initiated two phase 2 clinical trials to evaluate the possible reversal of liver fibrosis in NASH, the most common form of liver disease. The NASH-CX trial will focus on reversal of fibrosis in the most severe form of fibrosis called cirrhosis and the NASH-FX trial will attempt to show reversal of advanced fibrosis in a pre-cirrhotic stage.
More information on Galectin’s clinical trials can be found at clinicaltrials.gov
- NASH-CX: https://clinicaltrials.gov/ct2/show/NCT02462967?term=GR-MD-02&rank=3
- NASH-FX: https://clinicaltrials.gov/ct2/show/NCT02421094?term=GR-MD-02&rank=4
These “CEO Perspectives” will be a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may”, “could”, “expect” and others. These statements include those regarding the hope that Galectin’s development program for GR-MD-02 will lead to the first therapy for the treatment of fatty liver disease with advanced fibrosis and cirrhosis. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements
Please look for future editions in which multiple aspects of our development programs for unmet medical needs will be addressed.
Reference List
- Poynard T, Morra R, Halfon P, Castera L, Ratziu V, Imbert-Bismut F, et al. Meta-analyses of FibroTest diagnostic value in chronic liver disease. BMC Gastroenterol 2007;7:40.
- Atanasova E, Martinova F, Jelev D, Antonov K, de Mey C, Mateva L, et al. Alpha-2 macroglobulin is the simplest serum biomarker for liver fibrosis and fibrogenesis in chronic hepatitis C. MedInform 2015;1(2):153-164.
- Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le BB, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010 Feb;51(2):454-462.
Make a Comment or Ask a Question
[contact-form-7]
The post Successful Phase 1 Clinical Trial Supports Phase 2 Clinical Development Program appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Clinical Development Program in Liver Fibrosis appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>Galectin Therapeutics is developing a drug called GR-MD-02 which binds to and inhibits the galectin-3 protein which is important in the promotion of multiple types of disease including inflammation, fibrosis, and cancer. We have focused the use of this drug in a development program for potential treatment of patients with liver fibrosis, and specifically in those patients with NASH (non-alcoholic steatohepatitis) with advanced fibrosis or the most severe form of fibrosis, cirrhosis. There are currently no available therapies for liver fibrosis due to NASH.
A clinical development program includes a complex set of scientific experiments and human clinical trials that hopefully results in marketing approval of a drug for use in patients. One of the most important aspects of a clinical development program is specifying the indication, which guides all other decisions. In our case, we are focused on the treatment of NASH in two key target populations, NASH with cirrhosis, and NASH with advanced fibrosis, but not cirrhosis. The importance of the unmet medical need in these patients and the strength of the data from our preclinical studies with GR-MD-02 resulted in the FDA giving this development program Fast Track status. Our overall drug development program includes studies in all of the following areas:
- Pre-clinical (animal) studies to show efficacy in disease models
- Pre-clinical (animal) studies to evaluate for potential drug toxicity
- Pre-clinical (animal) studies to evaluate how the drug is distributed in the body, metabolized, and eliminated from the body
- Chemical development studies to characterize the structure and quality of the chemical or new molecular entity
- Process development studies to design and optimize the production processes
- Pharmaceutical or formulation development studies to design an appropriate form and route of administration for the final drug product
- Clinical Phase 1 studies to demonstrate safety in small numbers of healthy volunteers and patients with NASH with advanced fibrosis
- Clinical Phase 2 studies to establish efficacy and dose, as well as safety in the indicated patient population
- Clinical Phase 3 studies to confirm safety and efficacy for the intended marketing indication and target patient population
A great deal of work has already been completed on the development program for GR-MD-02. Pre-clinical animal studies have been completed that demonstrate efficacy in two different liver disease models and this work has been published in the peer-reviewed scientific literature (1, 2). Multiple studies have been done in animals to evaluate potential toxicity of GR-MD-02 and how the drug is handled in the body. Extensive work has been performed to establish the pharmaceutical quality of the drug and production processes. Several batches of consistent quality have been produced in accordance with FDA requirements for human use and administration. These studies provided the foundation for the FDA allowing us to move into human trials.
Human studies are divided into three phases. Phase 1 studies refer to the first human studies and are primarily to determine drug safety in a small number of patients, usually healthy volunteers. However, some Phase 1 trials are allowed to be performed in diseased patients in order to also evaluate whether the drug is having the intended effect. Phase 2 studies focus on evaluation of efficacy as well as safety of the drug in subjects with the target disease. This is the phase of clinical development when it is determined whether the drug doses studied may be effective in the disease indication. Phase 3 trials are larger studies that confirm the efficacy of the drug, evaluate safety in a larger patient population, and serve as the primary registration trials for marketing approval.
Galectin has completed two Phase 1 clinical trials, with one in NASH patients with advanced fibrosis and one in healthy volunteers. The Phase 1 trial in patients with NASH fibrosis was highly successful demonstrating safety, providing dosing information for phase 2, and showing an effect on markers of fibrosis. This will be the subject of its own “CEO Perspective” in the future. On the basis of these Phase 1 studies, Galectin has initiated two phase 2 clinical trials to evaluate the possible reversal of liver fibrosis in NASH, the most common form of liver disease. The NASH-CX trial will focus on reversal of fibrosis in the most severe form of fibrosis called cirrhosis and the NASH-FX trial will attempt to show reversal of advanced fibrosis in a pre-cirrhotic stage.
More information on Galectin’s clinical trials can be found at clinicaltrials.gov
- Phase 1 https://clinicaltrials.gov/ct2/show/NCT01899859?term=GR-MD-02&rank=2
- NASH-CX: https://clinicaltrials.gov/ct2/show/NCT02462967?term=GR-MD-02&rank=3
- NASH-FX: https://clinicaltrials.gov/ct2/show/NCT02421094?term=GR-MD-02&rank=4
These “CEO Perspectives” will be a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may”, “could”, “expect” and others. These statements include those regarding the hope that Galectin’s development program for GR-MD-02 will lead to the first therapy for the treatment of fatty liver disease with advanced fibrosis and cirrhosis. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements
Please look for future editions in which multiple aspects of our development programs for unmet medical needs will be addressed.
Reference List
- Traber PG, Chou H, Zomer E, Hong F, Klyosov A, Fiel MI, et al. Regression of fibrosis and reversal of cirrhosis in rats by galectin inhibitors in thioacetamide-induced liver disease. PLoS One 2013;8(10):e75361.
- Traber PG, Zomer E. Therapy of experimental NASH and fibrosis with galectin inhibitors. PLoS One 2013;8(12):e83481.
Make a Comment or Ask a Question
[contact-form-7]The post Clinical Development Program in Liver Fibrosis appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>