The post Fatty Liver is an Enormous Global Problem appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>Younossi and his colleagues collected data on over 8.5 million people from 22 countries, using a method called meta-analysis which allows combining data from multiple studies. (I have described this method in a previous Perspective.) Rinella and Charlton nicely summarized the main findings of this very important study in their editorial.
Before I describe the main findings, I must define some terms. Non-alcoholic fatty liver disease, or NAFLD, refers to abnormal accumulation of fat in liver cells. In some individuals, NAFLD progresses to NASH, or non-alcoholic steatohepatitis. In NASH, the presence of inflammation and dying liver cells is added to the accumulation of fat in liver cells. Some individuals with NASH have progressive fibrosis, or scarring of the liver, which may ultimately lead to cirrhosis, complications of cirrhosis, the need for liver transplant, and death.
The first conclusion of the study is that approximately one in four people in the world are affected by NAFLD. There are geographical differences in the rates of NAFLD across the world, as shown in the figure below published in the editorial (the numbers represent the percent of people in that region who have NAFLD).
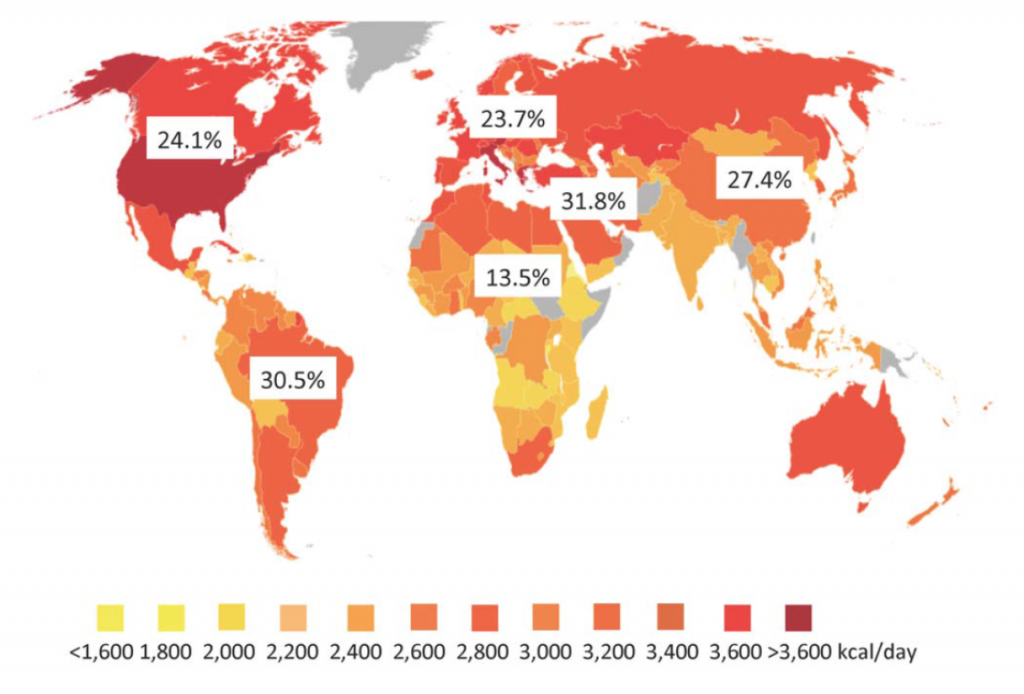
The color coding on this graph relates to the average caloric intake in the individual countries. While it is well known that obesity is associated with NAFLD, the relatively poor correlation with caloric intake with NAFLD rates suggests there are other factors involved. Some of these factors may include genetics (important in some regions), the type of food ingested, the composition of bacteria in the gut, and others.
The second major point taken from this study is that progression of fibrosis in NAFLD is slow and unusual. The editorial authors include an instructive figure to demonstrate this point. Out of 100 patients with NAFLD, only 5 will develop cirrhosis (the end stage of scarring) and only 1-2 patients will die as a result of that cirrhosis or require a liver transplant.
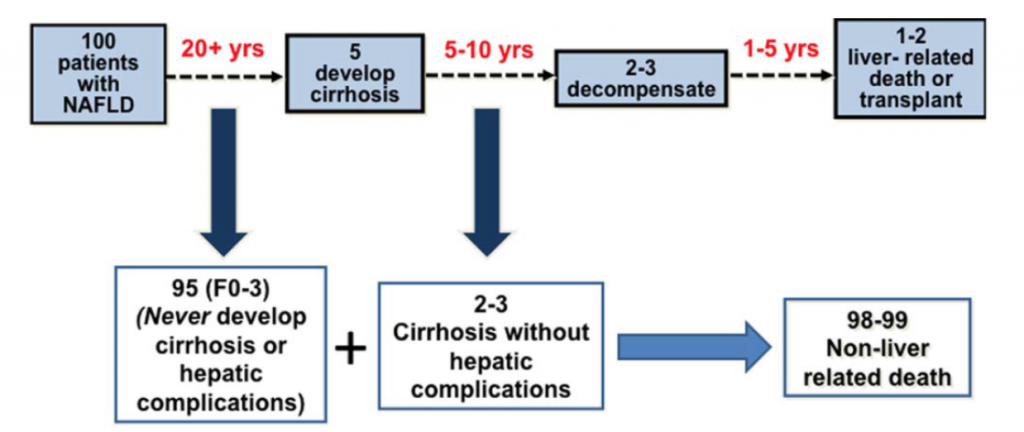
On face value, this seems like a small number of patients dying of their liver disease. However, when one considers the large number of people with NAFLD, those with cirrhosis represent a very significant problem. As stated by the authors, “Despite the low frequency of liver related mortality, the staggering denominator of over 1 billion adults with NAFLD coupled with a life-time risk of ~2% for liver-related mortality will eventually translate into ~20,000,000 liver-related deaths among patients currently alive with NAFLD.”
The final point is that NAFLD is, or will soon become, the most common cause of liver disease and liver-related death globally. The most important target for dealing with this enormous health problem is related to life-style changes to maintain a healthy liver, as I described in a previous Perspective. However, since there are currently no medicines available for this disease, we must also focus on those 20,000,000 people who are destined to die of NASH cirrhosis. This is the target for our Phase 2 clinical trial, NASH-CX, using our drug candidate GR-MD-02.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. For a discussion of factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2015, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Reference List
1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016 Jul;64(1):73-84.
2. Rinella M, Charlton M. The globalization of nonalcoholic fatty liver disease: Prevalence and impact on world health. Hepatology 2016 Jul;64(1):19-22.
Make a Comment or Ask a Question
[contact-form-7]The post Fatty Liver is an Enormous Global Problem appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Can Drinking Coffee Cure NASH or Liver Fibrosis? appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The short answer is, “Yes, drinking coffee does seem to reduce your chances of developing cirrhosis.” However, it’s not that simple so I wanted to dive into this news in a little more depth.
First, the relationship between coffee and cirrhosis is a topic that’s been examined in a number of different studies. For example, Stephen Harrison of Brooke Army Medical Center, one of the principal investigators in our NASH-FX trial and co-lead investigator in our NASH-CX trial, published a paper last year that showed an inverse relationship between coffee consumption and NASH activity, i.e., the more coffee you drank, the milder your NASH.
This most recent study was a meta-analysis of many different studies – a study of studies, if you will. These kinds of studies are very common, having been used to establish all things we know about smoking and lung cancer, for example, or cholesterol and heart disease. In fact, one of the studies used in the meta-analysis was from a heart study group; once you have characterized a large set of individuals for one purpose, you can look at the data generated by that study for other things as well.
There’s a formal, scientific process for undertaking this sort of research that involves culling the entire literature to find every single paper that examines a particular issue – in this case cirrhosis and coffee consumption – and then using a list of criteria to choose the best papers for combining in a meta-analysis. Nine studies met the criteria for this particular meta-analysis, resulting in a total studied population of more than 430,000 individuals. This is a very large sample size, which allows for greater confidence in the findings.
The evidence seems clear that coffee consumption is associated with lower rates of the development of cirrhosis. The study established a dose-response relationship based on the number of cups of coffee consumed per day. To summarize the findings in everyday language, if you increase your consumption of coffee by two cups per day, you decrease your likelihood of developing cirrhosis by half, which is a pretty profound effect.
However, there are many things this study doesn’t tell us: How long do you have to drink coffee to have this effect? At what age do you have to start? If you have liver disease, will drinking coffee help you? If you are out drinking alcohol, will ending the night with a couple of cups of coffee protect your liver? Does the brand or strength of the coffee make a difference? No, that’s not what was studied here (despite what the headline in CNN’s story might imply). It was simply an examination of how many people in this population have cirrhosis – or not –and how much coffee they say they drink. The relationship seems pretty strong, but this research doesn’t answer any of the other questions that people might have.
From a biochemical perspective it makes sense that coffee might have this effect. Many people tend to think of coffee as a dark liquid that has caffeine, but coffee is actually very complex, with hundreds of biologically active compounds. The vast majority of these compounds are taken up by the liver and are metabolized, just as the liver does with anything we eat and many drugs that we take.
This effect doesn’t extend to other caffeine-containing drinks. It appears to be the coffee that provides beneficial effects on the liver, not the caffeine. There have been studies comparing caffeinated with decaffeinated coffee, thereby isolating the effects of caffeine, and it appears likely that it’s not the caffeine that is the important factor. You can’t drink lots of Red Bull® and get the same effect.
What does this mean for patients and for physicians? To me, none of this precludes a common sense approach to liver health, as I published in a previous Perspective. However, coffee does seem to protect against liver disease, and there are other benefits that have been identified for coffee as well, including a reduction in all-cause mortality, neurological disease and some cancers, as recently reported for colon cancer. These benefits are balanced by some potential negative things about coffee. There are far fewer studies that suggest that coffee is bad for you, but there are some. For instance, there’s been a reported association with lung cancer. However, that effect is completely over-shadowed by tobacco use; people often smoke cigarettes while they’re drinking coffee, so it’s unclear that the coffee is to blame.
It’s possible we’ll never truly understand what it is about coffee that is responsible for this effect. There are people conducting experimental studies in animals and in cell lines, looking at the individual components of coffee in an attempt to determine which are having an effect on the fibrotic process. At some point, researchers may identify “compound X” within coffee, but we are a long way from turning this connection into something that would play an active role in the treatment of fibrosis and cirrhosis.
On balance, as a physician looking at these data, I think it makes sense to advise people who enjoy coffee to drink coffee. If coffee doesn’t bother you, and you’d like to have two or three cups a day, go ahead. It may benefit your liver, and there’s nothing like a good cup of coffee. That said, I wouldn’t think it right for a physician to start writing prescriptions for drinking coffee.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. For a discussion of factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2015, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Make a Comment or Ask a Question
[contact-form-7]The post Can Drinking Coffee Cure NASH or Liver Fibrosis? appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Love Your Liver: A Prescription for Liver Health appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>I am often asked how one can maintain a healthy liver and avoid the complications that can happen with chronic fatty liver disease. It has also recently been shown that fatty liver disease is an independent risk factor for cardiovascular disease, providing everyone with another important reason to maintain a healthy liver.
Liver health should be as important to everyone as heart health. However, how do you go about ensuring that your liver is healthy? I have listed below my suggested steps to maintain a healthy liver.
Maintain a healthy weight
There is a clear connection between body weight and the risk of NASH, including an increase in organ fat in apparently lean people (see here). If you are overweight, losing as little as 10% of your body weight can result in a much healthier liver (see here).
Follow a healthy diet
It is not my intention to provide specific dietary advice, but your liver diet should be preferably high in lean protein, low in carbohydrate like starch, low in added sugar, and limited high fructose corn syrup.
Exercise
Activity appears to improve fatty liver disease and help keep your liver healthy even if you don’t lose weight. Studies show resistance exercise is as beneficial as aerobic exercise.
Avoid excessive alcohol
Know the exact alcohol content of what you are drinking and what constitutes a “drink.” Men should drink a maximum of two drinks a day and women should limit themselves to one drink a day.
Avoid certain nutritional supplements
Certain nutritional supplements and herbal remedies can damage the liver. Talk to your doctor or read on-line about all supplements you are considering. For example, even too much iron or vitamin A can be harmful to your liver.
No illicit drug use
This may seem obvious, but you must avoid the use of drugs that expose you to blood from others, which put you at risk for hepatitis.
Discuss liver health with your doctor
Ask your primary care doctor, “How’s my liver?” You will often have gotten routine blood tests that include liver enzymes that may provide an indication of liver problems. Also, always ask whether there are potential liver effects from drugs, over-the-counter medicines, nutritional supplements and vitamins you may be taking.
These all seem like simple steps, but if everyone were following them we would not have the epidemic of fatty liver disease and other liver diseases we have today. Consider incorporating these steps in your lifestyle today.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. For a discussion of factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2015, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Make a Comment or Ask a Question
[contact-form-7]The post Love Your Liver: A Prescription for Liver Health appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Can Thin People Get Fatty Liver Disease? Lean NASH appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>I suppose it’s natural to assume that only overweight people get fatty liver disease (and NASH), but one thing I have learned over my career in medicine is to always challenge such “common sense” assumptions with empirical research. It turns out that, while people who are overweight or obese are indeed at greater risk, thin people can also develop fatty liver disease, NASH and cirrhosis. This has come to be known as “lean NASH.”
The commonly accepted definition of obesity is a body mass index (BMI) of 30 or more. However, overall BMI is less important in determining risk for fatty liver disease than where the fat is located in the body. Visceral fat, when the fat is nestled in and around the organs of the belly, is more strongly linked to fatty liver disease and other metabolic disorders than fat in arms, legs, and other parts of the body. A person can easily have a BMI well below 30 and still have considerable visceral fat. Someone who is “metabolically obese, normal weight” will have many of the hallmarks of obesity, such as insulin resistance (requirement of higher insulin levels to control blood sugar), metabolic syndrome (high blood sugar, elevated fats and cholesterol in blood, high blood pressure, and excess body fat around the waist) and NASH.
A study published in 2012 showed that overall prevalence of fatty liver disease among obese individuals was 28 percent, while fatty liver disease was identifiable in 7 percent of the lean individuals tested. (Zobair M. Younossi, 2012) Yes, obese people had a greater incidence of fatty liver disease, but lean people still showed a surprisingly high prevalence of the disease.
The other surprising finding from this study was that lean NASH patients here in the U.S. tend to be Hispanic. It’s unclear whether it is culture, diet, genetics, or some completely different mechanism at work that makes those of Hispanic ancestry more likely to develop lean NASH. But, given the growth of the Hispanic population here in the U.S., lean NASH is likely to emerge in the coming decades as an important cause of chronic liver disease.
Ethnicity does seem to be one of the major determinants of lean NASH, which was first described by physicians in Asia. While metabolic syndrome has long been a problem in developed countries, it is an increasing problem in developing countries as well, even though the rate of obesity remains comparatively low. The prevalence of fatty liver disease among normal-weight individuals was recently reported at 12% in Greece, 20% in India and 15% in China.
One analysis from 2013 suggests that lean NASH, as seen in Asia, is a distinct phenotype of NASH (Kausik Das, 2013). Asians, this study notes, show a propensity to develop metabolic syndrome at a lower BMI. One possible reason is that early malnutrition, either in utero or in early childhood, primes the body to store visceral fat more aggressively. The relative abundance of food in Asia today over the scarcities common only a few decades ago means that adults in China, India and other Asian countries are increasingly at risk of developing lean NASH.
I’ve called fatty liver disease a “hidden epidemic,” and lean NASH is even more so. It is easy to identify someone with a high body weight as being at risk for fatty liver disease, but less so for someone with lean NASH. We need a much better understanding of what causes lean NASH and how its presentation and biomarkers are distinct from the fatty liver disease and NASH seen in overweight patients.
Works Cited
- Kausik Das, A. C. (2013). Lean NASH: distinctiveness and clinical implication. Hepatol Int , 7(Supplement 2), S806 – S813.
- Zobair M. Younossi, M. M. (2012, November). Nonalcoholic Fatty Liver Disease in Lean Individuals in the United States. Medicine, 91(6), 319-227.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2015, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Make a Comment or Ask a Question
[contact-form-7]The post Can Thin People Get Fatty Liver Disease? Lean NASH appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post The Dilemma of Treating NASH appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>You may have seen Newsweek’s recent article on the growing impact of NASH, NASH Is the 21st Century’s Looming Health Threat. It’s a great article that vividly demonstrates the heartbreak of a NASH diagnosis and the shame and uncertainty faced by many patients.
“I’m in this situation and not for lack of good diet and exercise,” says Sandra, whose father died of liver disease 30 years ago. “You can blame me if you like, but I’m doing what I’m supposed to do.”
“Unlike those who suffer from other serious illnesses, NASH patients have no colored wristband denoting awareness, no annual fundraising race, no built-in support network.”
This article has gotten me thinking about ethical issues surrounding treating NASH and fatty liver disease.
There was a hint in the Newsweek article of the debate over whether medical intervention in NASH is at some level a questionable endeavor, as it may make better sense for society to address the epidemic of obesity directly rather than try and deal with NASH. However, that argument doesn’t hold up under scrutiny, as nearly 50% of the U.S. population is overweight or obese by the standard definitions. If you refused to treat medical problems that arise due to, or are exacerbated by obesity that would mean you wouldn’t treat heart disease, diabetes, or osteoarthritis. Even cancer is closely related to obesity and diet. Additionally, as I will elaborate on in a future CEO Perspective, lean people can also suffer from NASH so it is a lot more complicated than just a fat liver.
I wrote recently about how fatty liver disease helped motivate me to lose weight. I believe that awareness and lifestyle changes, including weight loss and exercise, will always be the best ways for the medical community and healthcare in general to address the looming health crisis of fatty liver disease. A recent study showed that a 10% reduction of body mass resolved NASH in 90% of the patients in the study.
However, I also remain convinced that there will always be a need for direct drug intervention for NASH. Not everyone will respond to a regime of weight loss and increased exercise. NASH is a progressive disease, and the question becomes: At what stage do we resort to direct medical intervention rather than focusing on lifestyle changes?
There was a study published last year in Hepatology [1] that took patients with a biopsy-proven diagnosis of NASH and followed them for up to 33 years, with an average of 26 years. This study was done in Sweden, so it was easier to track patients for that length of time than it might be here in the U.S. The study showed that if a patient started with stage 1 or 2 disease (mild fibrosis), they had no increased risk of mortality in comparison to a reference group. Let me repeat: Fatty liver disease itself didn’t affect patient mortality at all when followed for up to 33 years! However, if a patient had advanced fibrosis — stage 3 and 4 disease — they had a 3.3 times increase in mortality over the reference population.
To me, this study suggests that it would questionable to undertake a broad pharmaceutical approach to managing early-stage NASH. While a certain percentage of people will progress to advanced-stage NASH, we currently have no way of predicting which patient is at risk for this. Is it worth treating all 30 million people in the US with NASH, exposing them to the expense of the medication and its inevitable side effects when the treatment is not likely to have any impact on their long-term morbidity or mortality?
In contrast, we know that those who have NASH with advanced liver fibrosis do have an increase in morbidity and mortality related to NASH, so the more desirable approach might be to have a noninvasive test that can distinguish early-stage NASH from late-stage NASH and a follow-up treatment with a drug proven to be efficacious for late-stage NASH.
Galectin Therapeutics is distinctive among the companies developing therapies for treatment of NASH because we are focused exclusively on treatment of late-stage NASH with advanced fibrosis and cirrhosis. We have done this for the medical reasoning outlined here and the fact that the preclinical efficacy of our drug GR-MD-02 has a differentiated profile from other drugs in development in that it both prevents and reverses liver fibrosis. I think the science, the medical decision making, and ultimately the market will bear out that this is the point of greatest need.
- Journal of Hepatology, Volume 62, Supplement 2, April 2015, Pages S362–S363
Make a Comment or Ask a Question
[contact-form-7]The post The Dilemma of Treating NASH appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Portal hypertension and why it’s important appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>Everyone has had the experience of having their blood pressure taken and knows the importance of a normal reading for good health. But most people have never heard of liver blood pressure. In fact, high liver blood pressure, called portal hypertension, is the primary reason for complications and death in patients with advanced chronic liver disease, called cirrhosis.
The liver has a dual blood supply, which is different than most parts of the body. For example the kidney, intestines, muscles and many other tissues have a single blood supply that comes directly through arteries via the heart. The liver has two blood supplies — one directly from an artery (similar to other tissues), and a second blood flow from the portal vein. The greater proportion of blood comes from the portal vein, which drains the blood from the abdominal organs (stomach, small and large intestine, pancreas, and spleen).
Portal blood brings nutrients and hormones from the gastrointestinal tract which are important for liver function and homeostasis of many bodily functions. After arterial and portal blood mixes and flows through the liver, it returns to the heart via the main vein in the body.
Liver disease has a profound effect on this blood flow. All chronic liver disease leads to scar formation, or fibrosis. Whether the disease is due to a virus, alcohol, or fat, this scar tissue accumulates in the liver, with the most advanced stage called cirrhosis. The fibrotic tissue of cirrhosis distorts the liver architecture, impeding liver blood flow and, as a result, the blood pressure in the portal vein increases — this is called portal hypertension.
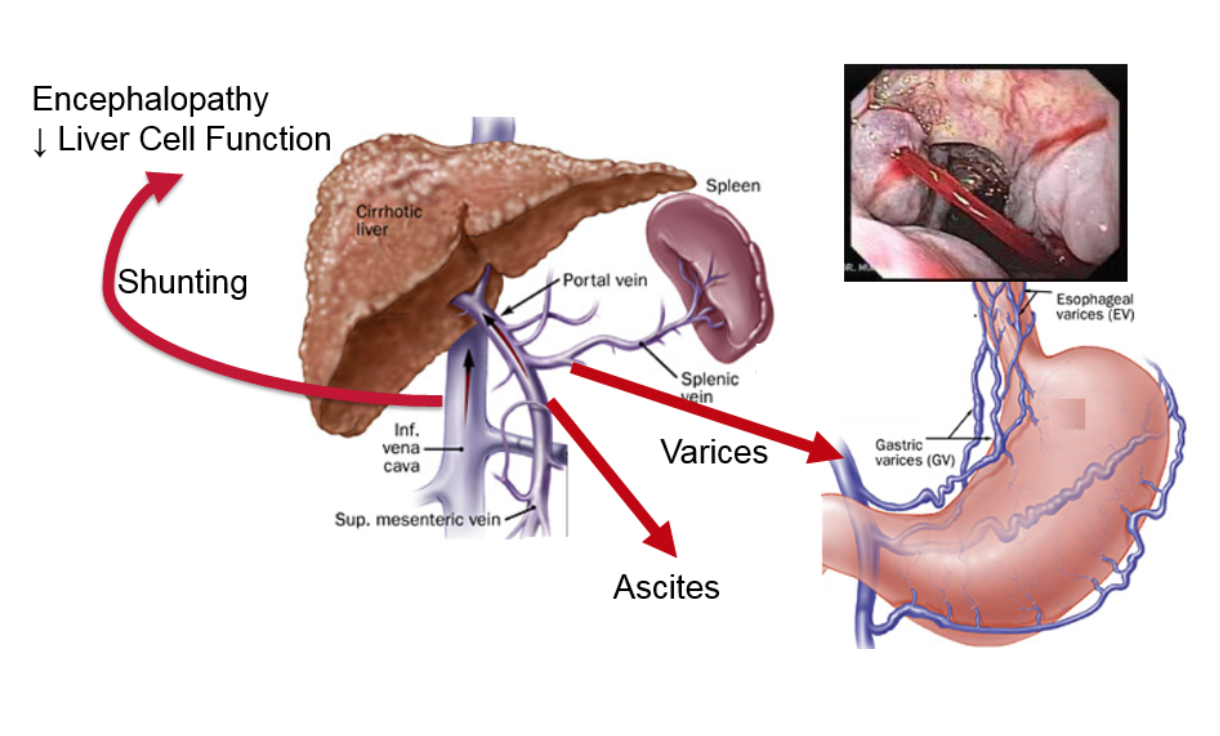
The figure shows what happens in portal hypertension. The increased pressure causes a certain type of vein dilations (similar to varicose veins or hemorrhoids), the most important of which are called esophageal varices. These dilated veins in the esophagus can burst and cause catastrophic bleeding. Another result of increased pressure is the build-up of fluid in the abdomen outside of the organs, called ascites, which is uncomfortable and can become infected. The increased pressure also results in additional vessels opening up, allowing blood to bypass the liver and enter directly into systemic circulation. Toxins that are normally removed by the liver gain direct access to the rest of the body and the brain, causing mental problems such as lethargy, confusion, and, in the worst cases, coma.
Portal hypertension does not occur with early stages of fibrosis but only when cirrhosis develops. In cirrhotic patients, the level of the portal pressure is directly related to the rate of cirrhotic complications and mortality. Additionally, if the portal pressure is decreased, the outcomes of patients are improved. This makes the portal pressure a potential surrogate for outcomes in clinical trials. If a drug therapy reduces portal pressure, it portends a better prognosis for the patient.
Measurement of portal pressure is not as simple as measuring systemic blood pressure. In fact, there is no direct way to measure portal pressure in clinical medicine. However, there is a minimally invasive radiology procedure that gives a very good estimate of portal pressure, called hepatic venous pressure gradient (HVPG). Rather than describe this technique, watch a short video of the procedure.
HVPG is the primary endpoint in our NASH-CX clinical trial, in which patients with cirrhosis are being treated with our galectin-3 inhibitor GR-MD-02 (see details of trial here). The goal of this trial is to reduce fibrosis in the liver, which will reduce the resistance to blood flow through the liver and, as a result, will reduce portal pressure and improve patient outcomes. It is important that we are also doing other tests for liver fibrosis and function, including liver biopsy (more here), FibroScan (more here), and the methacetin breath test (more here).
Measurement of HVPG to assess portal hypertension is a vital tool in evaluating potential therapies for liver cirrhosis. HVPG has been shown to be directly related to patient outcomes, making it a potential surrogate for drug registration trials.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Make a Comment or Ask a Question
[contact-form-7]The post Portal hypertension and why it’s important appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Developing Tests to Assess Liver Function in NASH and Cirrhosis appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>I wrote recently about the need for non-invasive tests to replace liver biopsies in the evaluation of liver fibrosis due to various diseases, including NASH, and I also spent time exploring some of the non-invasive serum and imaging tests that are in development. I started this whole discussion by stating that it is difficult to assess liver function and the effect of fibrosis on liver function. As a result, we must rely on direct physical assessments like biopsy and imaging tests and indirect evaluations like serum tests, which attempt to provide insight into what is going on in the liver. Yet there are a number of potential approaches to testing actual liver function being developed, and we are evaluating one of these in the course of our own clinical trials.
For example, one of the functions of the liver is to extract bile acids out of serum and excrete them into the bile that then enters the intestine. A company called HepQuant, headed by Dr. Greg Everson, whom I’ve known for many years, has developed a test for this. A small intravenous dose of a non-radioactive labeled bile acid and an oral dose are administered to the patient and the test then measures how well the liver takes up this bile acid.
HepQuant has generated data that demonstrate a linear correlation between its test’s results and various measures of liver disease, such as degree of fibrosis. Unfortunately, the test wasn’t ready for use when we started our clinical trials, but the HepQuant test could be a very important endpoint in the future. [1]
Another function of the liver is to metabolize drugs. A 13C-methacetin breath test developed by Exalenz Bioscience measures the metabolism of a drug, and we are making use of this test in our NASH CX trial. Developers have been pursuing this approach for many years, but it has only recently come into clinical use. [2]
The concept behind the 13C-methacetin breath test is that the liver metabolizes a drug called methacetin into acetaminophen. We’re probably all familiar with acetaminophen, which is sold over-the-counter as Tylenol®. Methacetin has been available for a long time as an oral drug for pain relief, but it’s not in high demand now because people tend to use acetaminophen as a first choice. In metabolizing methacetin, the liver uses an enzyme called cytochrome P450 1A1 to remove a methyl group off methacetin, which results in acetaminophen.
The amount of cytochrome P450 in the liver is related to liver disease, and the amount decreases as the severity of the disease increases. The liver has a tremendous capacity for metabolizing drugs, but with a sufficiently sensitive test, even small changes in the metabolic capacity can be identified before they become a big problem.
In the Exalenz 13C-methacetin breath test, the methyl group on the methacetin has been tagged with a carbon-13 (13C) atom, rather than the normal carbon-12 atom. You may recall from chemistry class that carbon-14 is a radioactive form of carbon, but carbon-13 is not. The 13C-laced methacetin is given to the patient orally. The liver metabolizes the methacetin by knocking off the methyl group, which is turned into 13C-laced CO2 and passes through the blood stream to be exhaled. The patient wears a nasal cannula and breathes normally, and a machine measures the amount of carbon-13 that is excreted. The more that is excreted, the better the liver is working.
This is a functional measure of the metabolic capacity of the liver. Exalenz has shown a correlation between liver damage and cirrhosis and the 13C-methacetin metabolic capacity. They’ve also shown that it is related to patient outcomes.
As these two tests are further refined and evaluated, they could become important in diagnosing and monitoring the treatment of liver disease, because they aren’t just looking at the structure of the liver, but they actually give us a sense of how liver function is changing.
Between serum, imaging and functional tests, there may soon be a wide range of reliable and non-invasive tests available to us. Each of these tests measures different things, so they’re likely to be used in combination. I can envision using an imaging test to look at the structure of the liver and a functional test to determine liver function. In our NASH-CX trial, that’s why we have the hepatic venous pressure gradient (HVPG), liver biopsy, FibroScan® and the 13C-methacetin breath test from Exalenz. I will discuss HVPG and why we are using this test in a future Perspective.
Our clinical trials are testing GR-MD-02, but at the same time we’re also evaluating the relevance of certain non-invasive tests. From my perspective, this is something that all companies developing treatments for NASH and liver fibrosis should incorporate into the design of their clinical trials. No matter what the results for the candidate drugs, we should also take the opportunity to gain a better understanding of how the results of non-invasive tests correlate to the results of a liver biopsy and disease progression. That would add tremendous momentum in this area of medicine and with improved diagnosis, it would support the commercial sales of these drugs when approved.
- HepQuant products are investigational combination drug and in vitro diagnostic devices and have not yet been evaluated or reviewed by the U.S. Food and Drug Administration (FDA). They are not currently available for clinical or investigational use or commercial sale.
- The Exalenz breath test is not currently approved in the U.S. for the assessment of liver function.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. These statements include those regarding the hope that Galectin Therapeutics’ development program for GR-MD-02 will show that it can be both safe and effective in the treatment of liver disease, including NASH. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Make a Comment or Ask a Question
[contact-form-7]The post Developing Tests to Assess Liver Function in NASH and Cirrhosis appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Fatty liver disease: Motivation to lose weight appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>Are you one of the nearly 40% of Americans classified as obese or are you overweight and inexorably headed towards obesity? Has your physician ever suggested you lose weight or have you made a New Year’s resolution to go on a diet? Do you need any more motivation to lose weight? If you do, here’s one: losing weight can reverse fatty liver disease and keep your liver healthy. And the good news is you don’t have to lose all that much weight to see a major improvement.
In fatty liver disease – also known as non-alcoholic steatohepatitis, or NASH – fat globules accumulate in liver cells, leading to the death of some of those cells and the development of an inflammatory reaction. With years of chronic inflammation, scar tissue begins to form in the liver via a process called fibrosis. When the scar tissue becomes severe, a condition called cirrhosis, the liver architecture becomes distorted and the blood flow to the liver is altered, resulting in life-threatening complications and liver failure.
The prevalence of NASH has reached epidemic proportions with as many as 25 million U.S. adults having the disease, as reported in a recent Newsweek article entitled “NASH is the 21st century’s looming public health threat.” I was interviewed for this article, and Galectin Therapeutics and our NASH therapeutic GR-MD-02 have a prominent place in the discussion. The article accurately reflects the critical aspects of this disease. Specifically, in its early stages with mild fibrosis, the disease can be improved with lifestyle changes including weight loss. However, when fibrosis is advanced, and particularly when cirrhosis is present, weight loss has much less effect and the only resort may be a liver transplant. This is why our drug treatment is focused on patients with advanced fibrosis and cirrhosis.
Now let’s get back to the good news. If you have early stage NASH – meaning you have inflammation with early stages of fibrosis – weight loss will significantly improve the health of your liver. In a recent clinical study, all patients who lost at least 10% of their body weight had reductions in their fatty liver disease on liver biopsy, with 90% having complete resolution of NASH. Additionally, patients who lost less weight, including as little as 3% of their body weight, also had significant improvements. In all patients who lost weight, every aspect of NASH was improved including fat in liver cells, liver cell death, and inflammation. It is important to note 61% of the patients in this study had no fibrosis, and it was mild in those that had fibrosis.
As described in the Newsweek article, I can personally affirm that weight loss can improve one’s liver. An ankle injury I suffered during a college football practice resulted in multiple surgeries and forced me to stop exercising, and I gained a significant amount of weight – in the neighborhood of 50 pounds. This resulted in high blood sugar and elevated liver enzymes, indicating potential damage to my liver due to fatty liver disease. My physician prescribed anti-diabetic medication, but I decided it was best to focus exclusively on losing weight. I was successful in losing approximately 10% of my body weight, and I’m continuing to lose.
While I am not yet at my ideal body weight, the improvements are dramatic. My blood glucose is now normal and stays normal throughout the day (and I’m not taking diabetes medication), and my liver enzymes have decreased and are now within the normal range. Also, I feel much better and my clothes fit! The important point is that you do not need to get all the way to your ideal weight to see dramatic improvements in liver health and other important health benefits. This is not an all-or-nothing proposition, and every little bit helps.
If you are one of those people carrying around extra weight, get started losing weight now. It doesn’t take much weight loss to improve your liver health. There are many approaches to losing weight, which you should discuss with your healthcare provider. And don’t forget to combine your weight loss program with exercise, which has also been shown to improve liver health. I will return to a number of the important issues raised in the Newsweek article in future Perspectives. In the meantime, I’ll see you at the salad bar.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. These statements include those regarding the hope that Galectin Therapeutic’ s development program for GR-MD-02 will show that it can be both safe and effective when used in combination with other drugs for the treatment of patients with cancer. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Footnotes:
- Defined as a BMI over 30; BMI = weight (in pounds) x 703 ÷ height (in inches) ÷ height (in inches)
- Available online as of January 30, 2015 and in the print edition dated February 12, 2016
Make a Comment or Ask a Question
[contact-form-7]The post Fatty liver disease: Motivation to lose weight appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Why Non-Invasive Testing is Needed in Liver Fibrosis appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>Several of my past CEO Perspectives have touched on the need for a non-invasive test in diagnosing and following treatment of fatty liver disease, non-alcoholic steatohepatitis (NASH), and cirrhosis. Currently, the only broadly accepted way to assess a patient’s condition is via a liver biopsy, an invasive method that is fraught with both potential side effects and inaccuracies. It would be a major benefit for physicians and patients if there was a simple, non-invasive test that would enable us to diagnose and track the progression of disease without resorting to biopsy.
There are a number of reasons why we lack a good non-invasive test for NASH and liver fibrosis. Some of them relate to the nature of the liver itself, others to the nature of the disease. In my next few blog posts, I’d like to explore these issues and review some of the more promising approaches for developing an effective non-invasive test for NASH and liver fibrosis.
The liver is different than other organs
There are characteristics of the liver that make it very different than other organs. For example, while the liver does many important things for the body, it’s hard to measure its functions. You can easily assess the function of the heart by measuring the heart rate and blood pressure, or the lungs by measuring the breathing rate, the oxygenation of the blood and asking the patient whether they are having shortness of breath. For the liver, it’s not easy to determine how it’s functioning from either physical exams or talking to the patient.
We also only have one liver, and it’s so important that it has a tremendous reserve capacity. You can knock out a high percentage of the liver and it still functions effectively to keep people alive. It’s a very resilient organ, and, for that reason, diseases can affect the liver for a long time before the liver function starts to fail. That’s one of the reasons why NASH patients can have inflammation in their liver and progressing fibrosis for decades with no or minimal symptoms and no adverse effects until late in the disease progress. Seemingly suddenly, they have cirrhosis.
These two characteristics – the lack of visibility to function and the large reserve capacity – make it difficult to assess liver function in chronic diseases. For instance, one of the functions of the liver is to remove hemoglobin pigments, which are formed into bilirubin and excreted by the liver into the gut. When this function backs up, it causes jaundice. Acute situations like acute viral hepatitis or a blocked bile duct will cause jaundice almost immediately. With a chronic liver disease like NASH, a patient won’t become jaundiced until very late in the disease. A large proportion of the liver can be compromised, and it can still excrete an adequate amount of bilirubin. The same holds true with the drug and glucose intermediary metabolism performed by the liver.
Why we rely on liver biopsy, and why we need something better
Because it is difficult to assess liver function, biopsy has been the mainstay for liver diagnosis for many years. Liver biopsy is often very important for assessing the cause of liver disease (etiology) and the extent of the disease (i.e., the degree of fibrosis). In fact, you cannot definitively determine whether somebody has NASH without doing a liver biopsy.
However, liver biopsies are invasive and are not without risk. The liver resides under the right diaphragm, and part of it is above the ribs and part of it below. In a liver biopsy, a needle is inserted between the ribs and then into the liver. This is a large needle, around twice the diameter of the needles used to draw blood. When the needle is pulled out, a core of liver tissue remains inside of the needle, and that is what is used for the evaluation. While a patient’s side and ribs will be numbed for the procedure, there is no way to numb the surface of the liver, so the biopsy procedure can be painful.
Then there are complications associated with performing the biopsy. The worst complication is bleeding. If the liver gets ripped and bleeds into the abdominal cavity, it can be catastrophic. The needle can also hit other things by mistake, such as the gall bladder. If the gall bladder leaks bile out into the abdomen, it could lead to emergent surgery. A liver biopsy is not a pleasant test, and there are potentially bad complications.
Despite our reliance on liver biopsies as the sole tool for definitive diagnosis of liver fibrosis, there are also potential problems in the accuracy of the findings, particular in assessing the extent of fibrosis. First, the biopsy tests only 1/50,000th of the liver, and the damage in liver fibrosis is usually heterogeneously distributed (patchy) throughout the liver. Given the large functional reserve of the liver, is it is difficult to be completely certain that such a small sample is representative of the overall health of the organ. Interpretation of the sample is also somewhat subjective. While many histological determinations are straightforward, determination of the degree of inflammation, bile duct damage and severity and location of fibrosis is left to the judgment of the pathologist.
Liver biopsies are now uncommon, but that may change
Relatively few liver biopsies are currently being performed in the U.S. Given that there is no current treatment for NASH, many doctors and patients decide that undergoing a liver biopsy is an unnecessary risk. A biopsy might confirm a diagnosis of NASH, but it will not change treatment at all.
As treatments do become available, we will need a better way of diagnosing NASH and importantly following its treatment. We have run into this issue in our NASH-CX clinical trial. We perform a liver biopsy of the participants at the beginning of treatment — to confirm a diagnosis of NASH and provide a baseline to compare against after treatment with GR-MD-02 — but it is simply too invasive and too risky to perform biopsies during the course of treatment. We are using other methods to evaluate progress during the studies, followed by a final biopsy at the end of the studies to confirm what was being observed.
In future CEO Perspectives, I will explore some of the non-invasive tests that are being developed.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. These statements include those regarding the hope that Galectin Therapeutic’ s development program for GR-MD-02 will show that it can be both safe and effective when used in combination with other drugs for the treatment of patients with cancer. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Make a Comment or Ask a Question
[contact-form-7]The post Why Non-Invasive Testing is Needed in Liver Fibrosis appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post 2015 – 2016, Progress and Possibilities appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The many accomplishments at Galectin Therapeutics during 2015 ranged from incremental progress touching every aspect of our business to significant advancements with GR-MD-02. Importantly, this progress forms the basis for numerous milestones expected in the coming years including clinical progress, intellectual property fortification, further engagement with the investment community and ongoing outreach to educate our shareholders about our work to develop new therapies, the regulatory environment in which we operate and our target markets.
CEO Perspectives, Dr. Traber’s blog introduced last year, is designed to provide scientific and technical information largely regarding our work with GR-MD-02 in layman’s language. Much of our progress and accomplishments during 2015 were chronicled in the 14 blog postings, which can be found here.
We were delighted that in the final days of 2015 a U.S. District Court dismissed both the federal securities class-action lawsuit and the shareholder derivative actions lawsuit filed in the Summer of 2014 against Galectin and certain officers, directors and a shareholder, which had cast an inappropriate cloud over our many achievements in 2015. The Court entered final judgments of dismissals in both actions based on the Court’s finding that any further amendment of the complaints would be futile (i.e., dismissed with prejudice). Plaintiffs have the right to appeal the Court’s dismissals within 30 days. Based on the Federal Court’s rulings, Galectin is seeking dismissal of a duplicative shareholder derivative action in Nevada which was filed after the federal actions.
Our Clinical Programs
NASH with advanced liver fibrosis
Development of GR-MD-02 for the treatment of non-alcoholic steatohepatitis (NASH) with advanced fibrosis and cirrhosis continues to be the primary focus of our company. We completed a successful Phase 1 clinical trial and announced final data in January 2015. The Phase 1 trial demonstrated that GR-MD-02 is safe, with potential for therapeutic effect on fibrosis in NASH patients with advanced fibrosis. We found no serious adverse events and no treatment-emergent adverse events related to our drug. Furthermore, GR-MD-02 was found to be safe and well tolerated in each of the three dose-escalating cohorts of patients, who were suffering from NASH with advanced fibrosis.
This finding alone defines the study as a success, but we gained additional valuable information. We found that the FibroTest® score, a composite biomarker of five different blood tests that has been correlated with the extent of liver fibrosis, was significantly reduced by GR-MD-02 treatment in the third dosing cohort of 8 mg/kg. In addition, we found that some patients in this cohort also showed a decrease in liver stiffness, which has a direct correlation with fibrosis. We published a comprehensive piece on the results of the Phase 1 study in a CEO Perspectives blog post, which can be found here.
In addition to the phase 1 trial in NASH patients with advanced fibrosis, we reported the results of a drug-drug interaction study with GR-MD-02 and midazolam, a common sedative, which showed that in healthy volunteers there was no unfavorable interaction between the two compounds. Because many patients with chronic diseases are on multiple medications over long periods of time and may take other medications on an intermittent basis, this finding is important to the commercial potential of GR-MD-02 and to the patient population that is eligible to participate in our Phase 2 program. We published a CEO Perspectives piece on this topic, which is available here.
The information gleaned from the Phase 1 studies formed the basis for our Phase 2 program, which consists of two studies, one in NASH patients with advanced fibrosis and the other in NASH patients with cirrhosis. We submitted our protocol to the U.S. Food and Drug Administration (FDA) for the cirrhosis study in the first quarter, engaged our contract research organization and began screening patients at the end of June.
This study, the NASH-CX trial, is a multicenter, randomized, placebo-controlled, double-blind, parallel-group Phase 2 trial to evaluate the safety and efficacy of GR-MD-02 for the treatment of liver fibrosis and resultant portal hypertension (HVPG) in patients with NASH cirrhosis. A total of 156 patients at approximately 50 sites in the U.S. will be randomized to receive either 2 mg/kg of GR-MD-02, 8 mg/kg of GR-MD-02 or placebo, with 52 patients in each arm. The primary endpoint is a reduction in HVPG. Patients will receive a total of 26 infusions every other week for one year, at which time they will be evaluated for change in HVPG compared with placebo. HVPG will be correlated with secondary endpoints of fibrosis on liver biopsy as well as with measurement of liver stiffness via FibroScan® and assessment of liver metabolism (13C-methacetin breath test, Exalenz), which are non-invasive measures of the liver that may be used in future studies. More information can be found at www.clinicaltrials.gov and in a CEO Perspectives blog post, which can be found here.
We are pleased with the pace of the NASH-CX study and we remain on track to provide data readout in at the end of 2017, as we have previously indicated.
In September we initiated a 30-patient study with GR-MD-02 in NASH patients with advanced fibrosis, our NASH-FX study, with 15 patients receiving 8 mg/kg of GR-MD-02 and 15 patients receiving placebo every other week for 16 weeks. This study will evaluate the safety and efficacy of GR-MD-02 on liver fibrosis using multi-parametric magnetic resonance imaging (LiverMultiScan®, Perspectum Diagnostics) as the primary endpoint and liver stiffness as assessed by magnetic resonance-elastography and FibroScan as secondary endpoints. This study is also proceeding as planned, with top-line data expected around the end of the third quarter of 2016. We published a CEO Perspectives piece on this trial, which is available here.
Psoriasis
As we have previously reported, one of the patients participating in our Phase 1 NASH study was a long-term psoriasis sufferer, and this patient’s psoriasis cleared as the study progressed, and remained cleared for many months following the conclusion of the study. With an established theoretical pathway for how inhibition of galectin-3 might affect psoriasis, in September we began an open label 10-patient Phase 2a pilot study in patients with moderate-to-severe plaque psoriasis. We expect data readout from this study late in the third quarter of 2016. More information and background on this study can be found here.
Melanoma
We continued to support independent research with GR-MD-02 in combination with two commercial melanoma drugs, as preclinical research has shown our compound enhances the efficacy of immune checkpoint blockade therapies, or so-called checkpoint inhibitors, a new class of drugs. GR-MD-02 is progressing through a Phase 1b study in combination with Yervoy®, and a Phase 1b study in combination with Keytruda® was initiated in the fourth quarter of 2015 with enrollment to begin early in 2016. Preclinical work in mouse cancer models with GR-MD-02 added to checkpoint inhibitors shows a boost in anti-tumor immunity, a reduction in tumor size and increased survival. Both of these studies are being conducted at the Providence Cancer Center in Portland, Oregon. Galectin is providing GR-MD-02 to the investigators, who are funding the costs of these studies. We published a CEO Perspectives on these trials, which is available here.
In the trial combining Yervoy and GR-MD-02, two dosing cohorts have been completed and the third cohort delivering 4 mg/kg of GR-MD-02 is enrolling now. Of the seven patients that have received the combination therapy, there has been no dose limiting toxicity. Following completion of the 4 mg/kg dose cohort, a total of 10 patients will be dosed at 8 mg/kg. Immune markers as well as tumor response are being monitored in this study.
Significant Presentations and Publications
Galectin’s researchers presented at several important industry meetings during the year. Dr. Traber delivered an invited presentation of the company’s research with GR-MD-02 in NASH at the American Association for the Study of Liver Diseases (AASLD) Industry Colloquium in March. He participated in the session entitled “NASH: Clinical Endpoints and Drug Development” and discussed the role of galectin-3 in organ fibrosis generally and liver fibrosis in particular, and GR-MD-02 as a galectin-3 inhibitor. He reviewed the published preclinical data showing that GR-MD-02 is effective in reversing inflammation and fibrosis in a mouse model of NASH and also in reversing cirrhosis and improving portal hypertension in a rat model of cirrhosis. He also reviewed the company’s Phase 1 study results and its Phase 2 clinical program design. The abstract of Dr. Traber’s presentation can be found here.
In addition, preclinical research from a study led by Stefanie Linch, Ph.D. in the laboratory of tumor immunology expert William L. Redmond, Ph.D. of the Providence Cancer Center’s Earle A. Chiles Research Institute was presented in November at the Society for Immunotherapy of Cancer’s (SITC) 30th Anniversary Annual Meeting. The studies presented were conducted by the Institute in collaboration with Galectin Therapeutics. The poster presentation “Galectin-3 inhibition using novel inhibitor GR-MD-02 improves survival and immune function while reducing tumor vasculature” and an abstract was published in the Journal for ImmunoTherapy of Cancer. The poster presentation is available for review here.
Interviews with Dr. Traber appeared in a number of publications throughout 2015, including R&D Magazine in a piece entitled “Finding the Holy Grail Treatment for Fatty Livers,” available here, Obesity News Today published its Q&A article entitled, “Exclusive: Dr. Peter Traber Discusses Non-alcoholic Fatty Liver Disease”, available here, and MD Magazine, which conducted an online interview with Dr. Traber at the AASLD meeting. That interview can be found here.
Galectin management also participated in a number of investment conferences throughout the year, including programs for institutional investors, retail investors and family offices.
Foundational Support for our Business
During 2015 we considerably strengthened our intellectual property portfolio and received a U.S. patent Notice of Allowance for the use of pectin compounds to reduce fibrosis in multiple diseases. This patent is particularly important because it not only permits GR-MD-02 use for NASH with fibrosis, but it covers other compounds in our pipeline and a multitude of diseases with a fibrotic etiology. We also continued to build our international patent portfolio with patents issued or allowed in Israel and Australia.
We also made excellent progress with our Chemistry, Manufacturing and Controls (CMC), all of which are vital to the proper conduct of our clinical trials with GR-MD-02 and are essential components of the final application to the FDA for a drug’s approval. We discussed this progress during the year in the CEO Perspective post found here. We also reached a very significant milestone in our preclinical toxicology program, having completed chronic administration of GR-MD-02 in two animal species, allowing chronic administration in human subjects.
Lastly, we were very pleased to have completed a $9.8 million financing during the fourth quarter. This capital is expected to fund currently planned operations through the first quarter of 2017, and will be used mainly for clinical trial expenses and other research and development expenses, as well as for general corporate purposes.
Looking Ahead to 2016
We are looking forward to executing on several important milestones in 2016, with highlights including the following:
- We will continue enrollment in the NASH-CX study and work with our investigators and contract research organization to keep on our stated timelines for data readout in late 2017
- We will continue enrollment in the NASH-FX study, and continue to expect data readout around the end of the third quarter of 2016
- We will continue enrollment in the psoriasis Phase 2a study, with data readout also expected at the end of the third quarter of 2016
- While we do not control the rate of enrollment of the trial, we expect data from the Providence Cancer Center’s study with Yervoy in combination with GR-MD-02 in advanced metastatic melanoma by the end of 2016.
- We expect that Providence Cancer Center will be enrolling patients in the study with GR-MD-02 in combination with Keytruda during 2016.
We are fully aware that yesterday’s accomplishments set tomorrow’s expectations, and we look forward to creating shareholder value by executing on numerous milestones during 2016. We are grateful to our long-standing, loyal shareholders for their continued support and to the hard-working staff at Galectin Therapeutics who share a unifying commitment to addressing significant unmet clinical needs in NASH, as well as in oncology and psoriasis.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. These statements include those regarding the hope that Galectin Therapeutic’ s development program for GR-MD-02 will show that it can be both safe and effective when used in combination with other drugs for the treatment of patients with cancer. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements
Make a Comment or Ask a Question
[contact-form-7]The post 2015 – 2016, Progress and Possibilities appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>