The post Portal hypertension and why it’s important appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>Everyone has had the experience of having their blood pressure taken and knows the importance of a normal reading for good health. But most people have never heard of liver blood pressure. In fact, high liver blood pressure, called portal hypertension, is the primary reason for complications and death in patients with advanced chronic liver disease, called cirrhosis.
The liver has a dual blood supply, which is different than most parts of the body. For example the kidney, intestines, muscles and many other tissues have a single blood supply that comes directly through arteries via the heart. The liver has two blood supplies — one directly from an artery (similar to other tissues), and a second blood flow from the portal vein. The greater proportion of blood comes from the portal vein, which drains the blood from the abdominal organs (stomach, small and large intestine, pancreas, and spleen).
Portal blood brings nutrients and hormones from the gastrointestinal tract which are important for liver function and homeostasis of many bodily functions. After arterial and portal blood mixes and flows through the liver, it returns to the heart via the main vein in the body.
Liver disease has a profound effect on this blood flow. All chronic liver disease leads to scar formation, or fibrosis. Whether the disease is due to a virus, alcohol, or fat, this scar tissue accumulates in the liver, with the most advanced stage called cirrhosis. The fibrotic tissue of cirrhosis distorts the liver architecture, impeding liver blood flow and, as a result, the blood pressure in the portal vein increases — this is called portal hypertension.
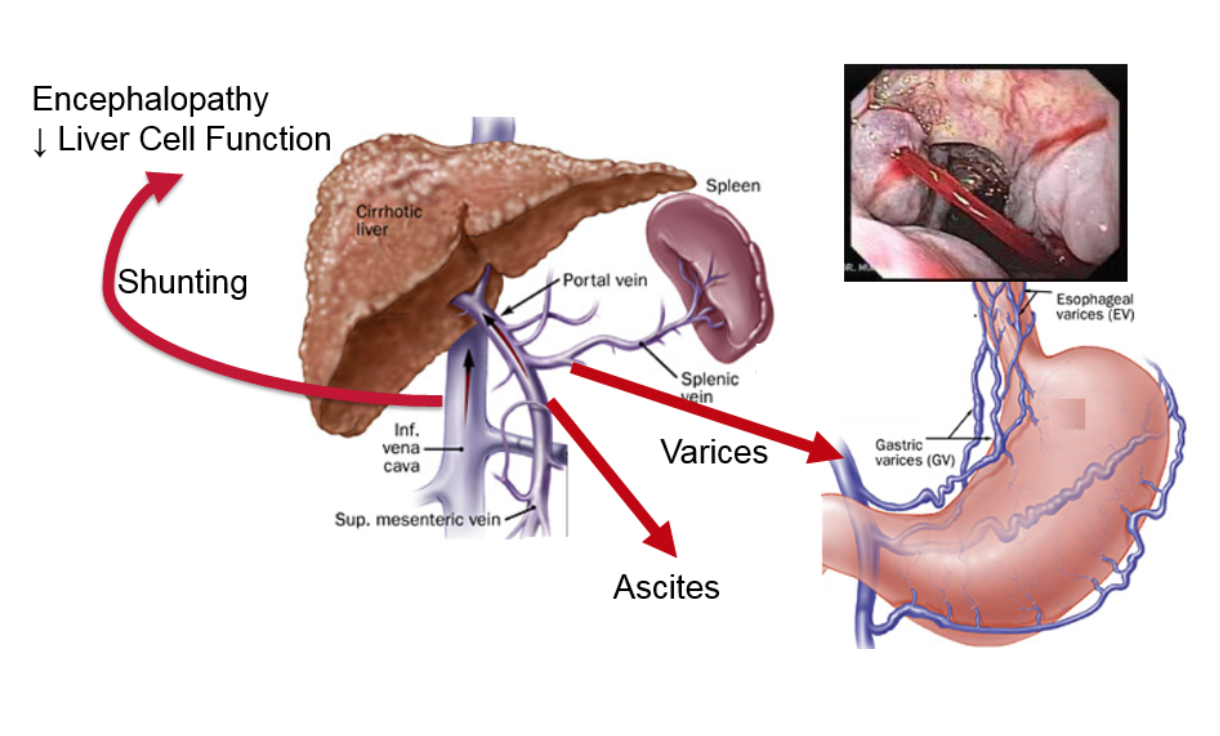
The figure shows what happens in portal hypertension. The increased pressure causes a certain type of vein dilations (similar to varicose veins or hemorrhoids), the most important of which are called esophageal varices. These dilated veins in the esophagus can burst and cause catastrophic bleeding. Another result of increased pressure is the build-up of fluid in the abdomen outside of the organs, called ascites, which is uncomfortable and can become infected. The increased pressure also results in additional vessels opening up, allowing blood to bypass the liver and enter directly into systemic circulation. Toxins that are normally removed by the liver gain direct access to the rest of the body and the brain, causing mental problems such as lethargy, confusion, and, in the worst cases, coma.
Portal hypertension does not occur with early stages of fibrosis but only when cirrhosis develops. In cirrhotic patients, the level of the portal pressure is directly related to the rate of cirrhotic complications and mortality. Additionally, if the portal pressure is decreased, the outcomes of patients are improved. This makes the portal pressure a potential surrogate for outcomes in clinical trials. If a drug therapy reduces portal pressure, it portends a better prognosis for the patient.
Measurement of portal pressure is not as simple as measuring systemic blood pressure. In fact, there is no direct way to measure portal pressure in clinical medicine. However, there is a minimally invasive radiology procedure that gives a very good estimate of portal pressure, called hepatic venous pressure gradient (HVPG). Rather than describe this technique, watch a short video of the procedure.
HVPG is the primary endpoint in our NASH-CX clinical trial, in which patients with cirrhosis are being treated with our galectin-3 inhibitor GR-MD-02 (see details of trial here). The goal of this trial is to reduce fibrosis in the liver, which will reduce the resistance to blood flow through the liver and, as a result, will reduce portal pressure and improve patient outcomes. It is important that we are also doing other tests for liver fibrosis and function, including liver biopsy (more here), FibroScan (more here), and the methacetin breath test (more here).
Measurement of HVPG to assess portal hypertension is a vital tool in evaluating potential therapies for liver cirrhosis. HVPG has been shown to be directly related to patient outcomes, making it a potential surrogate for drug registration trials.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Make a Comment or Ask a Question
[contact-form-7]The post Portal hypertension and why it’s important appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post The Potential for Treatment of Liver Fibrosis With Galectin’s Drug Candidate GR-MD-02 appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>Liver fibrosis is scar formation in the liver which, when it reaches a large amount of scar tissue (called cirrhosis), results in liver failure and complications leading to death. The only available treatment for liver cirrhosis is a transplant, which is not only expensive, but is limited in supply (just over 6,000 annually in U.S.). Many liver diseases lead to fibrosis, including hepatitis, alcohol abuse, and fatty liver disease, which is called NASH (non-alcoholic steatohepatitis). Galectin is focused on liver fibrosis due to NASH because it is the most common liver disease and there are currently no available therapies.
Fatty liver disease, or NASH, can take decades to progress to advanced fibrosis and cirrhosis and we cannot predict which patients with early NASH disease will progress to advanced fibrosis. Galectin is encouraged by its galectin inhibitor, GR-MD-02, because it both reduces the inflammation of NASH and, importantly, can reverse liver fibrosis in pre-clinical animal models. The evidence for this has been published in peer-reviewed scientific articles (1, 2). Many companies in the NASH space are focused on early disease because the mechanism of their drug likely does not have a direct effect on fibrosis. In our opinion, our drug has a broader potential particularly in late stage NASH because GR-MD-02 has, in pre-clinical studies as reported in the above peer-reviewed articles, reversed advanced fibrosis and cirrhosis. In addition to not knowing which patients will progress to advanced fibrosis, a drug targeting early stage NASH is likely to require chronic administration for many years, thus possibly treating some patients unnecessarily, along with taxing the healthcare system with increased costs.
The medical community has encouraged our continued research as evidenced by references to our therapy in scientific publications. For example, Galectin Therapeutics’ compound that targets galectin-3 protein, GR-MD-02, is discussed in Expert Reviews in Gastroenterology and Hepatology, “In search of the magic bullet: can liver inflammation and fibrosis be reversed with medication?” (3) In their review of several potential mechanisms, the authors state that galectin-3 is “emerging as a possible target to revert liver fibrosis” and cite Galectin Therapeutics’ peer reviewed publication on preclinical data showing reversal of cirrhosis in an animal model and phase 1 clinical trial results.
Another recent reference is found in a review in Clinics and Research in Hepatology and Gastroenterology entitled “Novel therapeutic targets for steatohepatitis (4).” Arun Sanyal, M.D., of the Virginia Commonwealth University School of Medicine states, “Galectin has been targeted for the treatment of NASH with advanced fibrosis…Preliminary studies have confirmed its safety and tolerability. Phase 2 studies to clarify its efficacy are now under way.”
We are pleased that experts in the field have recognized our drug compound, GR-MD-02, as having potential in both the inflammation of NASH and in reversing advanced fibrosis. Galectin has initiated two phase 2 clinical trials to evaluate the possible reversal of liver fibrosis in NASH, the most common form of liver disease. The NASH-CX trial will focus on reversal of fibrosis in the most severe form of fibrosis called cirrhosis and the NASH-FX trial will attempt to show reversal of advanced fibrosis in a pre-cirrhotic stage.
These “CEO Perspectives” will be a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may”, “could”, “expect” and others. These statements include those regarding the hope that Galectin’s development program for GR-MD-02 will lead to the first therapy for the treatment of fatty liver disease with advanced fibrosis and cirrhosis. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements
Please look for future editions in which multiple aspects of our development programs for unmet medical needs will be addressed.
Reference List
- Traber PG, Chou H, Zomer E, Hong F, Klyosov A, Fiel MI, et al. Regression of fibrosis and reversal of cirrhosis in rats by galectin inhibitors in thioacetamide-induced liver disease. PLoS One 2013;8(10):e75361.
- Traber PG, Zomer E. Therapy of experimental NASH and fibrosis with galectin inhibitors. PLoS One 2013;8(12):e83481.
- Zimmerman HW, Tacke F. In search of the magic bullet: can liver inflammation and fibrosis be reversed with medications? Expert Rev Gastroenterol Hepatol 2015;1-3.
- Sanyal AJ. Novel therapeutic targets for steatohepatitis. Clin Res Hepatol Gastroenterol 2015 Jul 6.
Make a Comment or Ask a Question
[contact-form-7]The post The Potential for Treatment of Liver Fibrosis With Galectin’s Drug Candidate GR-MD-02 appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>