The post Fatty Liver is an Enormous Global Problem appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>Younossi and his colleagues collected data on over 8.5 million people from 22 countries, using a method called meta-analysis which allows combining data from multiple studies. (I have described this method in a previous Perspective.) Rinella and Charlton nicely summarized the main findings of this very important study in their editorial.
Before I describe the main findings, I must define some terms. Non-alcoholic fatty liver disease, or NAFLD, refers to abnormal accumulation of fat in liver cells. In some individuals, NAFLD progresses to NASH, or non-alcoholic steatohepatitis. In NASH, the presence of inflammation and dying liver cells is added to the accumulation of fat in liver cells. Some individuals with NASH have progressive fibrosis, or scarring of the liver, which may ultimately lead to cirrhosis, complications of cirrhosis, the need for liver transplant, and death.
The first conclusion of the study is that approximately one in four people in the world are affected by NAFLD. There are geographical differences in the rates of NAFLD across the world, as shown in the figure below published in the editorial (the numbers represent the percent of people in that region who have NAFLD).
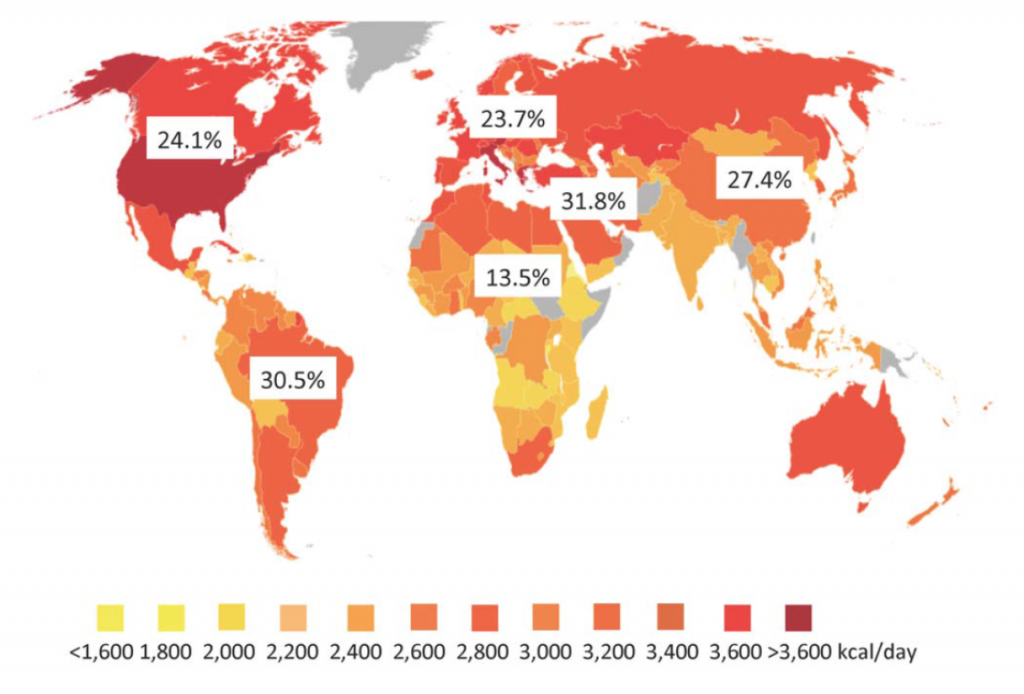
The color coding on this graph relates to the average caloric intake in the individual countries. While it is well known that obesity is associated with NAFLD, the relatively poor correlation with caloric intake with NAFLD rates suggests there are other factors involved. Some of these factors may include genetics (important in some regions), the type of food ingested, the composition of bacteria in the gut, and others.
The second major point taken from this study is that progression of fibrosis in NAFLD is slow and unusual. The editorial authors include an instructive figure to demonstrate this point. Out of 100 patients with NAFLD, only 5 will develop cirrhosis (the end stage of scarring) and only 1-2 patients will die as a result of that cirrhosis or require a liver transplant.
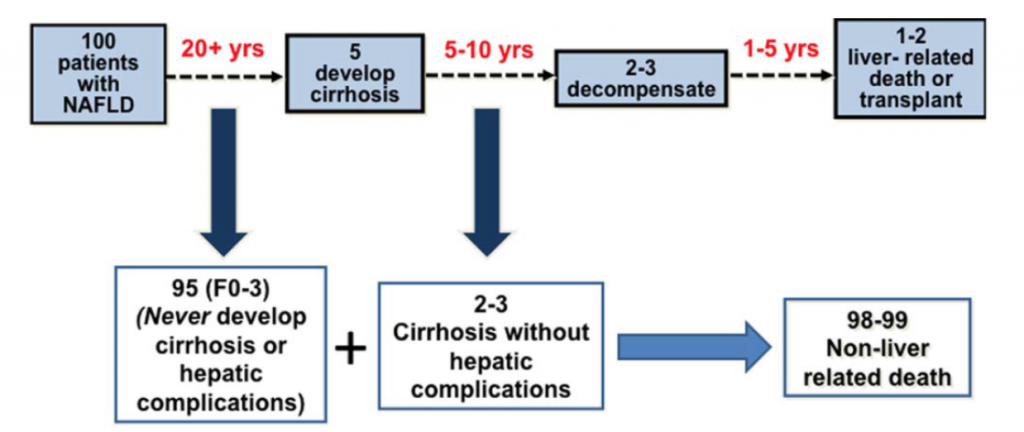
On face value, this seems like a small number of patients dying of their liver disease. However, when one considers the large number of people with NAFLD, those with cirrhosis represent a very significant problem. As stated by the authors, “Despite the low frequency of liver related mortality, the staggering denominator of over 1 billion adults with NAFLD coupled with a life-time risk of ~2% for liver-related mortality will eventually translate into ~20,000,000 liver-related deaths among patients currently alive with NAFLD.”
The final point is that NAFLD is, or will soon become, the most common cause of liver disease and liver-related death globally. The most important target for dealing with this enormous health problem is related to life-style changes to maintain a healthy liver, as I described in a previous Perspective. However, since there are currently no medicines available for this disease, we must also focus on those 20,000,000 people who are destined to die of NASH cirrhosis. This is the target for our Phase 2 clinical trial, NASH-CX, using our drug candidate GR-MD-02.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. For a discussion of factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2015, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Reference List
1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016 Jul;64(1):73-84.
2. Rinella M, Charlton M. The globalization of nonalcoholic fatty liver disease: Prevalence and impact on world health. Hepatology 2016 Jul;64(1):19-22.
Make a Comment or Ask a Question
[contact-form-7]The post Fatty Liver is an Enormous Global Problem appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Love Your Liver: A Prescription for Liver Health appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>I am often asked how one can maintain a healthy liver and avoid the complications that can happen with chronic fatty liver disease. It has also recently been shown that fatty liver disease is an independent risk factor for cardiovascular disease, providing everyone with another important reason to maintain a healthy liver.
Liver health should be as important to everyone as heart health. However, how do you go about ensuring that your liver is healthy? I have listed below my suggested steps to maintain a healthy liver.
Maintain a healthy weight
There is a clear connection between body weight and the risk of NASH, including an increase in organ fat in apparently lean people (see here). If you are overweight, losing as little as 10% of your body weight can result in a much healthier liver (see here).
Follow a healthy diet
It is not my intention to provide specific dietary advice, but your liver diet should be preferably high in lean protein, low in carbohydrate like starch, low in added sugar, and limited high fructose corn syrup.
Exercise
Activity appears to improve fatty liver disease and help keep your liver healthy even if you don’t lose weight. Studies show resistance exercise is as beneficial as aerobic exercise.
Avoid excessive alcohol
Know the exact alcohol content of what you are drinking and what constitutes a “drink.” Men should drink a maximum of two drinks a day and women should limit themselves to one drink a day.
Avoid certain nutritional supplements
Certain nutritional supplements and herbal remedies can damage the liver. Talk to your doctor or read on-line about all supplements you are considering. For example, even too much iron or vitamin A can be harmful to your liver.
No illicit drug use
This may seem obvious, but you must avoid the use of drugs that expose you to blood from others, which put you at risk for hepatitis.
Discuss liver health with your doctor
Ask your primary care doctor, “How’s my liver?” You will often have gotten routine blood tests that include liver enzymes that may provide an indication of liver problems. Also, always ask whether there are potential liver effects from drugs, over-the-counter medicines, nutritional supplements and vitamins you may be taking.
These all seem like simple steps, but if everyone were following them we would not have the epidemic of fatty liver disease and other liver diseases we have today. Consider incorporating these steps in your lifestyle today.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. For a discussion of factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2015, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Make a Comment or Ask a Question
[contact-form-7]The post Love Your Liver: A Prescription for Liver Health appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Can Thin People Get Fatty Liver Disease? Lean NASH appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>I suppose it’s natural to assume that only overweight people get fatty liver disease (and NASH), but one thing I have learned over my career in medicine is to always challenge such “common sense” assumptions with empirical research. It turns out that, while people who are overweight or obese are indeed at greater risk, thin people can also develop fatty liver disease, NASH and cirrhosis. This has come to be known as “lean NASH.”
The commonly accepted definition of obesity is a body mass index (BMI) of 30 or more. However, overall BMI is less important in determining risk for fatty liver disease than where the fat is located in the body. Visceral fat, when the fat is nestled in and around the organs of the belly, is more strongly linked to fatty liver disease and other metabolic disorders than fat in arms, legs, and other parts of the body. A person can easily have a BMI well below 30 and still have considerable visceral fat. Someone who is “metabolically obese, normal weight” will have many of the hallmarks of obesity, such as insulin resistance (requirement of higher insulin levels to control blood sugar), metabolic syndrome (high blood sugar, elevated fats and cholesterol in blood, high blood pressure, and excess body fat around the waist) and NASH.
A study published in 2012 showed that overall prevalence of fatty liver disease among obese individuals was 28 percent, while fatty liver disease was identifiable in 7 percent of the lean individuals tested. (Zobair M. Younossi, 2012) Yes, obese people had a greater incidence of fatty liver disease, but lean people still showed a surprisingly high prevalence of the disease.
The other surprising finding from this study was that lean NASH patients here in the U.S. tend to be Hispanic. It’s unclear whether it is culture, diet, genetics, or some completely different mechanism at work that makes those of Hispanic ancestry more likely to develop lean NASH. But, given the growth of the Hispanic population here in the U.S., lean NASH is likely to emerge in the coming decades as an important cause of chronic liver disease.
Ethnicity does seem to be one of the major determinants of lean NASH, which was first described by physicians in Asia. While metabolic syndrome has long been a problem in developed countries, it is an increasing problem in developing countries as well, even though the rate of obesity remains comparatively low. The prevalence of fatty liver disease among normal-weight individuals was recently reported at 12% in Greece, 20% in India and 15% in China.
One analysis from 2013 suggests that lean NASH, as seen in Asia, is a distinct phenotype of NASH (Kausik Das, 2013). Asians, this study notes, show a propensity to develop metabolic syndrome at a lower BMI. One possible reason is that early malnutrition, either in utero or in early childhood, primes the body to store visceral fat more aggressively. The relative abundance of food in Asia today over the scarcities common only a few decades ago means that adults in China, India and other Asian countries are increasingly at risk of developing lean NASH.
I’ve called fatty liver disease a “hidden epidemic,” and lean NASH is even more so. It is easy to identify someone with a high body weight as being at risk for fatty liver disease, but less so for someone with lean NASH. We need a much better understanding of what causes lean NASH and how its presentation and biomarkers are distinct from the fatty liver disease and NASH seen in overweight patients.
Works Cited
- Kausik Das, A. C. (2013). Lean NASH: distinctiveness and clinical implication. Hepatol Int , 7(Supplement 2), S806 – S813.
- Zobair M. Younossi, M. M. (2012, November). Nonalcoholic Fatty Liver Disease in Lean Individuals in the United States. Medicine, 91(6), 319-227.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2015, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Make a Comment or Ask a Question
[contact-form-7]The post Can Thin People Get Fatty Liver Disease? Lean NASH appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Fatty liver disease: Motivation to lose weight appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>Are you one of the nearly 40% of Americans classified as obese or are you overweight and inexorably headed towards obesity? Has your physician ever suggested you lose weight or have you made a New Year’s resolution to go on a diet? Do you need any more motivation to lose weight? If you do, here’s one: losing weight can reverse fatty liver disease and keep your liver healthy. And the good news is you don’t have to lose all that much weight to see a major improvement.
In fatty liver disease – also known as non-alcoholic steatohepatitis, or NASH – fat globules accumulate in liver cells, leading to the death of some of those cells and the development of an inflammatory reaction. With years of chronic inflammation, scar tissue begins to form in the liver via a process called fibrosis. When the scar tissue becomes severe, a condition called cirrhosis, the liver architecture becomes distorted and the blood flow to the liver is altered, resulting in life-threatening complications and liver failure.
The prevalence of NASH has reached epidemic proportions with as many as 25 million U.S. adults having the disease, as reported in a recent Newsweek article entitled “NASH is the 21st century’s looming public health threat.” I was interviewed for this article, and Galectin Therapeutics and our NASH therapeutic GR-MD-02 have a prominent place in the discussion. The article accurately reflects the critical aspects of this disease. Specifically, in its early stages with mild fibrosis, the disease can be improved with lifestyle changes including weight loss. However, when fibrosis is advanced, and particularly when cirrhosis is present, weight loss has much less effect and the only resort may be a liver transplant. This is why our drug treatment is focused on patients with advanced fibrosis and cirrhosis.
Now let’s get back to the good news. If you have early stage NASH – meaning you have inflammation with early stages of fibrosis – weight loss will significantly improve the health of your liver. In a recent clinical study, all patients who lost at least 10% of their body weight had reductions in their fatty liver disease on liver biopsy, with 90% having complete resolution of NASH. Additionally, patients who lost less weight, including as little as 3% of their body weight, also had significant improvements. In all patients who lost weight, every aspect of NASH was improved including fat in liver cells, liver cell death, and inflammation. It is important to note 61% of the patients in this study had no fibrosis, and it was mild in those that had fibrosis.
As described in the Newsweek article, I can personally affirm that weight loss can improve one’s liver. An ankle injury I suffered during a college football practice resulted in multiple surgeries and forced me to stop exercising, and I gained a significant amount of weight – in the neighborhood of 50 pounds. This resulted in high blood sugar and elevated liver enzymes, indicating potential damage to my liver due to fatty liver disease. My physician prescribed anti-diabetic medication, but I decided it was best to focus exclusively on losing weight. I was successful in losing approximately 10% of my body weight, and I’m continuing to lose.
While I am not yet at my ideal body weight, the improvements are dramatic. My blood glucose is now normal and stays normal throughout the day (and I’m not taking diabetes medication), and my liver enzymes have decreased and are now within the normal range. Also, I feel much better and my clothes fit! The important point is that you do not need to get all the way to your ideal weight to see dramatic improvements in liver health and other important health benefits. This is not an all-or-nothing proposition, and every little bit helps.
If you are one of those people carrying around extra weight, get started losing weight now. It doesn’t take much weight loss to improve your liver health. There are many approaches to losing weight, which you should discuss with your healthcare provider. And don’t forget to combine your weight loss program with exercise, which has also been shown to improve liver health. I will return to a number of the important issues raised in the Newsweek article in future Perspectives. In the meantime, I’ll see you at the salad bar.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. These statements include those regarding the hope that Galectin Therapeutic’ s development program for GR-MD-02 will show that it can be both safe and effective when used in combination with other drugs for the treatment of patients with cancer. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Footnotes:
- Defined as a BMI over 30; BMI = weight (in pounds) x 703 ÷ height (in inches) ÷ height (in inches)
- Available online as of January 30, 2015 and in the print edition dated February 12, 2016
Make a Comment or Ask a Question
[contact-form-7]The post Fatty liver disease: Motivation to lose weight appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Are You Eligible for the Galectin Therapeutics’ NASH Cirrhosis Trial? appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>In June 2015 Galectin Therapeutics initiated a clinical trial to determine if our investigational drug GR-MD-02 can successfully treat patients with severe scarring of the liver (cirrhosis) due to fatty liver disease (a.k.a. nonalcoholic steatohepatitis, or NASH). The goal of the study (the NASH-CX trial) is to assess whether GR-MD-02 may reduce the fibrous tissue that is clogging the liver in subjects with NASH cirrhosis. A reduction, in turn, is expected to improve liver function and have a positive effect on patient outcomes, such as perhaps delaying or avoiding a liver transplant. Please see my earlier CEO Perspective, “Clinical Trial to Establish Efficacy of GR-MD-02 in NASH Cirrhosis,” where this study is described in more detail.
Can you participate in this clinical trial?
If your cirrhosis is caused by NASH and you have portal hypertension, but you haven’t developed other complications related to your cirrhosis, then you might be eligible to participate in this trial. You must be between 18 and 75 years old and meet various other study eligibility requirements. This study is limited to people with cirrhosis only caused by NASH and not by any other factors, like alcohol or hepatitis.
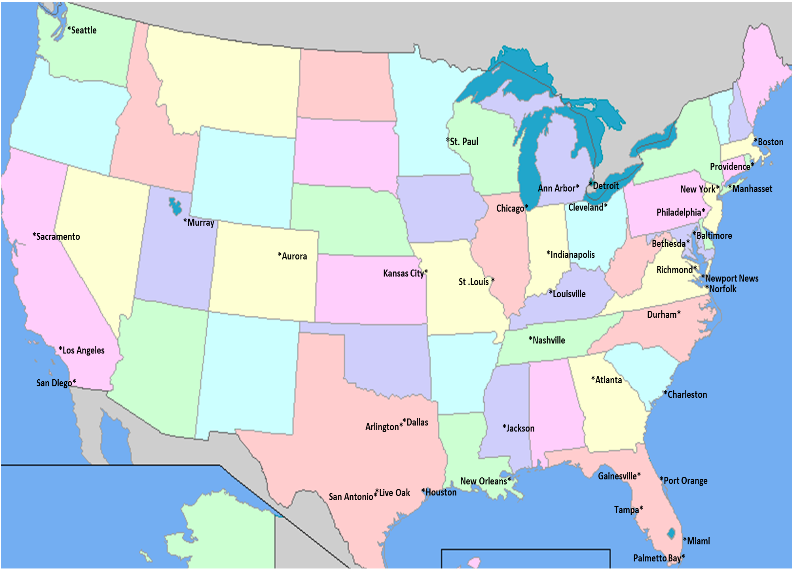
If you qualify, you would be one of 156 participants at about 45 to 60 study sites, or clinics, across the United States. It is important to remember that this is an experimental study comparing GR-MD-02 against a placebo; the trial design is such that you have 2 out of 3 chances of being on active drug and 1 out of 3 chances of being treated with placebo.
The study medication will be administered by an intravenous infusion every two weeks for one year. We expect that each clinic visit will last about an hour and a half. Below you will find a map showing the cities where the study is being conducted to see if you are able to make it to the site every two weeks. If you think you might be eligible for our study, please contact one of the study sites (click here for the list of sites and the appropriate contact information).
More information about this trial can be found at clinicaltrials.gov:
• NASH-CX: https://clinicaltrials.gov/ct2/show/NCT02462967?term=GR-MD-02&rank=3
Should the NASH-CX trial be successful, this will be the first clinical study of an investigational drug candidate to show a reduction of fibrosis in people with cirrhosis and the first hope of a future approved treatment other than a liver transplant.
These “CEO Perspectives” will be a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “could,” “expect” and others. These statements include those regarding the hope that Galectin’s development program for GR-MD-02 will lead to the first therapy for the treatment of fatty liver disease with advanced fibrosis and cirrhosis. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements
Please look for future editions in which multiple aspects of our development programs for unmet medical needs will be addressed.
GT-026 STUDY SITE MAP
Make a Comment or Ask a Question
[contact-form-7]The post Are You Eligible for the Galectin Therapeutics’ NASH Cirrhosis Trial? appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Lessons Learned from the Failure of Raptors’ Phase 2b NASH Trial appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>On September 14, 2015, Raptor Pharmaceutical Corp. (NASDAQ: RPTP) announced top-line results of its Phase 2b trial evaluating RP103 in children with biopsy-confirmed nonalcoholic steatohepatitis (NASH). As reported here, the study failed to meet its primary endpoint of a two-point decrease in a fatty-liver-disease scoring system and no worsening of fibrosis, or scarring of the liver. It’s always disappointing when a promising drug candidate fails in a clinical trial, especially when it addresses something like NASH, for which there really aren’t any proven treatment options.
The failure of the Raptor trial provides an important lesson for any company developing therapies to address fatty liver disease and NASH on the use of surrogate blood markers in tracking the progression (or regression) of fatty liver disease and NASH.
If you’ve ever had routine blood work done as part of a physical, the tests probably included the measurement of the transaminases AST (aspartate aminotransferase) and ALT (alanine aminotransferase). These are enzymes that naturally circulate in the blood. They are also enzymes that are in cells and, in particular, they are in liver cells. If there is damage to the liver, as with a viral hepatitis A infection, these enzymes will be released into the blood serum in great numbers. A marked increase in the transaminases in your blood is one of the ways doctors diagnose hepatitis and other liver diseases.
Transaminases are often elevated in fatty liver disease and NASH. If you recruit a group of NASH patients, on average, the liver enzymes are going to be elevated. It’s easy to make the logical conclusion that if you have a drug that might work in NASH, and you treat those patients, the transaminase levels should come down. A number of trials have been undertaken with that presumption, and one of them was Raptor’s.
Between 2008 and 2010, Raptor conducted a small, open-label trial (meaning there wasn’t a placebo arm in the trial) with its drug – a long-acting, slow-release cysteamine – in adolescents with NASH (mean age 14). Eleven patients completed the trial and seven patients had either normalization or >50% reduction of their transaminase (ALT) level from baseline. These results led the investigators to suspect the drug was having activity in NASH. They quickly moved on to the latest Phase 2b study; Raptor even received the support of the National Institutes of Health (NIH), which agreed to conduct and help fund the trial.
In this newest study, the transaminase levels of the patients indeed went down, but liver biopsies showed no change in the histology of that organ. This is evidence that the changes in transaminase levels seen in NASH patients are not necessarily indicative of the activity of the underlying disease. While there may be a correlation with some treatments, this trial shows that this is not always the case. In reality, patients with fatty liver disease and NASH will see their levels of transaminases fluctuate widely. Roughly two-thirds of all patients, at any given time, will have normal transaminases. There’s no good evidence that transaminases correlate with either the stage or the activity of the disease.
This failure of the Raptor study also has a broad implication for all clinical trials involving NASH because it demonstrates that serum transaminases cannot be relied upon as an early biomarker of the effectiveness of a drug. It may also have ramifications for other potential biomarkers. The lesson from Raptor’s failure shows that we need to be skeptical about serum biomarkers until they’re well correlated with changes in the liver itself.
Here at Galectin we are ever mindful of the need to correlate biomarkers with disease and how difficult the task is. In our Phase 2 program in NASH with advanced fibrosis and cirrhosis, we are performing multiple tests to ensure we have correlation with the underlying disease. In the NASH-CX trial we are performing both invasive (including biopsy) and non-invasive physical and metabolic tests to evaluate correlation (see discussion here) and the NASH-FX trials utilizes three of the most promising methods for non-invasive imaging methods to assess physical properties of the liver that correlate with fibrosis (see discussion here).
While not every clinical trial ends with success, they all help advance our understanding of fatty liver disease and NASH. It’s just a matter of learning the lessons they teach us.
These “CEO Perspectives” will be a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “could,” “expect” and others. These statements include those regarding the hope that Galectin’s development program for GR-MD-02 will lead to an additional therapy for the treatment of psoriasis. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements
Please look for future editions in which multiple aspects of our development programs for unmet medical needs will be addressed.
Make a Comment or Ask a Question
[contact-form-7]The post Lessons Learned from the Failure of Raptors’ Phase 2b NASH Trial appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Second Phase 2 clinical trial initiated for fatty liver disease with advanced fibrosis appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>As a company, we are focused on developing a therapy for patients with non-alcoholic steatohepatitis (NASH), or fatty liver disease, with advanced fibrosis (scarring) of the liver. Our drug GR-MD-02 has been shown to prevent and reverse fibrosis in preclinical animal models and there was evidence of a therapeutic effect in our Phase 1 trial (see previous CEO Perspectives). With the recent start of our second Phase 2 clinical trial (see September 16, 2015 press release), we now have studies underway that address two distinct NASH patient populations including those with cirrhosis, the most advanced stage of fibrosis, and those with advanced fibrosis without cirrhosis.
The newly announced NASH-FX clinical trial, discussed here, will attempt to show reversal of advanced fibrosis in a pre-cirrhotic stage. The goal is to reduce fibrosis to an early stage, slowing or preventing the patient from progressing to cirrhosis. Therefore, the NASH-FX trial addresses a very different patient population than the NASH-CX trial which treats NASH cirrhosis (see previous CEO Perspectives), which could lead to a separate indication.
The NASH-FX trial will treat NASH patients with advanced fibrosis with 8 mg/kg of GR-MD-02 or placebo for four months (see diagram below). The Principal Investigator, a prominent liver physician, and we believe that this is a sufficiently long period of time to show an effect on fibrosis given the results and treatment time of the Phase 1 trial. Additional trial details can be found at: https://clinicaltrials.gov/ct2/show/NCT02421094?term=GR-MD-02&rank=4
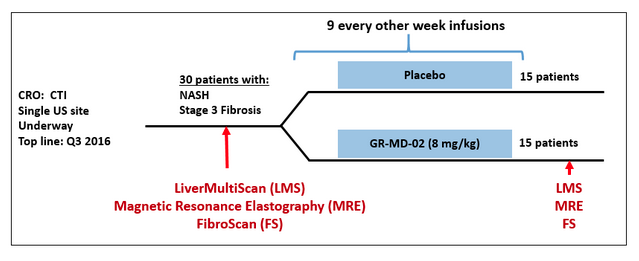
In the NASH-FX trial we will be using non-invasive imaging tests of the liver to assess fibrosis. The most definitive test for liver fibrosis involves microscopic evaluation of liver tissue obtained via a biopsy. The NASH-CX trial, for example, is using liver biopsy as one of the multiple evaluations of fibrosis in that trial. However, the short duration of the NASH-FX trial precludes repeated liver biopsies in patients because of safety concerns and potential discomfort related to the biopsies.
Liver biopsy is not a perfect test, as interpretation of liver biopsies is fraught with inconsistencies due to sampling error since the tissue piece obtained only represents approximately 1/50,000th of the liver. These inconsistencies along with the risk of the procedure itself have led investigators across the world to seek better, non-invasive approaches to evaluate liver fibrosis. One approach is to evaluate blood tests that may reflect the state of fibrosis in the liver, but this has not yet yielded results that are useful in clinical trials.
Better results have been obtained with methods to image the liver and assess physical characteristics of the liver tissue that is reflective of fibrosis. In the NASH-FX trial, we are using three of the most promising methods for directly assessing physical properties of the liver that correlate with fibrosis. The primary endpoint will be an assessment of fibrosis over the entire liver using multi-parametric magnetic resonance imaging (LiverMultiScan®), which is a validated and proprietary MRI protocol of Perspectum Diagnostics. Published information shows that this method is a sensitive approach to demonstrate differences in the degree of liver fibrosis (1). We chose this as the primary endpoint because data from Perspectum Diagnostics shows there are only small variations in repeated tests in the same individual.
Secondary endpoints will evaluate liver stiffness, which correlates to the degree of liver fibrosis, as assessed by magnetic resonance-elastography (MRE) and FibroScan® (FS). MRE provides a measure of liver stiffness over the entire imaged organ and has high diagnostic accuracy for detection of fibrosis in NASH, independent of BMI and degree of inflammation (2, 3). FS provides a measure of stiffness in a region of liver tissue about 100 times larger than a liver biopsy and also has good diagnostic accuracy for detecting liver fibrosis (4). These three tests will provide information on drug effect, as well as on the performance of the three tests relative to one another, which may indicate the best test(s) to utilize in future studies.
How will the results of the NASH-FX trial advance GR-MD-02 toward approval? With successful improvement in non-invasive fibrosis assessments, we will have proof-of-concept that treatment with GR-MD-02 over four months may be useful in this patient population. Because we are using actual physical liver measurements that correlate with fibrosis, positive study results will significantly improve the odds of success with future clinical trials and inform the dose and duration of treatment moving forward. While this trial alone will not be sufficient for drug approval for this indication, it will be critical for the design of pivotal trials for the purpose of marketing approval. We anticipate results for the NASH-FX trial will be available in the third quarter of 2016.
These “CEO Perspectives” will be a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “could,” “expect” and others. These statements include those regarding the hope that Galectin’s development program for GR-MD-02 will lead to the first therapy for the treatment of fatty liver disease with advanced fibrosis and cirrhosis. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements
Please look for future editions in which multiple aspects of our development programs for unmet medical needs will be addressed.
Reference List
1. Banerjee R, Pavlides M, Tunnicliffe EM, Piechnik SK, Sarania N, Philips R, et al. Multiparametric magnetic resonance for the non-invasive diagnosis of liver disease. J Hepatol 2014 Jan;60(1):69-77.
2. Singh S, Venkatesh SK, Loomba R, Wang Z, Sirlin C, Chen J, et al. Magnetic resonance elastography for staging liver fibrosis in non-alcoholic fatty liver disease: a diagnostic accuracy systematic review and individual participant data pooled analysis. Eur Radiol 2015 Aug 28.
3. Singh S, Venkatesh SK, Wang Z, Miller FH, Motosugi U, Low RN, et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: a systematic review and meta-analysis of individual participant data. Clin Gastroenterol Hepatol 2015 Mar;13(3):440-451.
4. Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le BB, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010 Feb;51(2):454-462.
Make a Comment or Ask a Question
[contact-form-7]The post Second Phase 2 clinical trial initiated for fatty liver disease with advanced fibrosis appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>