The post Second Phase 2 clinical trial initiated for fatty liver disease with advanced fibrosis appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>As a company, we are focused on developing a therapy for patients with non-alcoholic steatohepatitis (NASH), or fatty liver disease, with advanced fibrosis (scarring) of the liver. Our drug GR-MD-02 has been shown to prevent and reverse fibrosis in preclinical animal models and there was evidence of a therapeutic effect in our Phase 1 trial (see previous CEO Perspectives). With the recent start of our second Phase 2 clinical trial (see September 16, 2015 press release), we now have studies underway that address two distinct NASH patient populations including those with cirrhosis, the most advanced stage of fibrosis, and those with advanced fibrosis without cirrhosis.
The newly announced NASH-FX clinical trial, discussed here, will attempt to show reversal of advanced fibrosis in a pre-cirrhotic stage. The goal is to reduce fibrosis to an early stage, slowing or preventing the patient from progressing to cirrhosis. Therefore, the NASH-FX trial addresses a very different patient population than the NASH-CX trial which treats NASH cirrhosis (see previous CEO Perspectives), which could lead to a separate indication.
The NASH-FX trial will treat NASH patients with advanced fibrosis with 8 mg/kg of GR-MD-02 or placebo for four months (see diagram below). The Principal Investigator, a prominent liver physician, and we believe that this is a sufficiently long period of time to show an effect on fibrosis given the results and treatment time of the Phase 1 trial. Additional trial details can be found at: https://clinicaltrials.gov/ct2/show/NCT02421094?term=GR-MD-02&rank=4
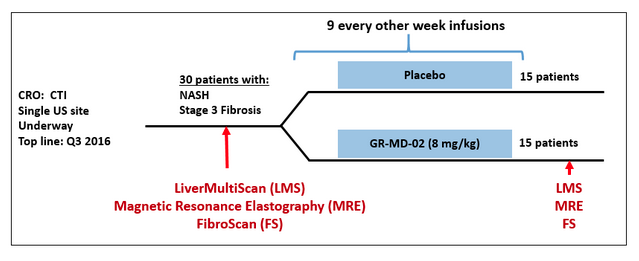
In the NASH-FX trial we will be using non-invasive imaging tests of the liver to assess fibrosis. The most definitive test for liver fibrosis involves microscopic evaluation of liver tissue obtained via a biopsy. The NASH-CX trial, for example, is using liver biopsy as one of the multiple evaluations of fibrosis in that trial. However, the short duration of the NASH-FX trial precludes repeated liver biopsies in patients because of safety concerns and potential discomfort related to the biopsies.
Liver biopsy is not a perfect test, as interpretation of liver biopsies is fraught with inconsistencies due to sampling error since the tissue piece obtained only represents approximately 1/50,000th of the liver. These inconsistencies along with the risk of the procedure itself have led investigators across the world to seek better, non-invasive approaches to evaluate liver fibrosis. One approach is to evaluate blood tests that may reflect the state of fibrosis in the liver, but this has not yet yielded results that are useful in clinical trials.
Better results have been obtained with methods to image the liver and assess physical characteristics of the liver tissue that is reflective of fibrosis. In the NASH-FX trial, we are using three of the most promising methods for directly assessing physical properties of the liver that correlate with fibrosis. The primary endpoint will be an assessment of fibrosis over the entire liver using multi-parametric magnetic resonance imaging (LiverMultiScan®), which is a validated and proprietary MRI protocol of Perspectum Diagnostics. Published information shows that this method is a sensitive approach to demonstrate differences in the degree of liver fibrosis (1). We chose this as the primary endpoint because data from Perspectum Diagnostics shows there are only small variations in repeated tests in the same individual.
Secondary endpoints will evaluate liver stiffness, which correlates to the degree of liver fibrosis, as assessed by magnetic resonance-elastography (MRE) and FibroScan® (FS). MRE provides a measure of liver stiffness over the entire imaged organ and has high diagnostic accuracy for detection of fibrosis in NASH, independent of BMI and degree of inflammation (2, 3). FS provides a measure of stiffness in a region of liver tissue about 100 times larger than a liver biopsy and also has good diagnostic accuracy for detecting liver fibrosis (4). These three tests will provide information on drug effect, as well as on the performance of the three tests relative to one another, which may indicate the best test(s) to utilize in future studies.
How will the results of the NASH-FX trial advance GR-MD-02 toward approval? With successful improvement in non-invasive fibrosis assessments, we will have proof-of-concept that treatment with GR-MD-02 over four months may be useful in this patient population. Because we are using actual physical liver measurements that correlate with fibrosis, positive study results will significantly improve the odds of success with future clinical trials and inform the dose and duration of treatment moving forward. While this trial alone will not be sufficient for drug approval for this indication, it will be critical for the design of pivotal trials for the purpose of marketing approval. We anticipate results for the NASH-FX trial will be available in the third quarter of 2016.
These “CEO Perspectives” will be a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “could,” “expect” and others. These statements include those regarding the hope that Galectin’s development program for GR-MD-02 will lead to the first therapy for the treatment of fatty liver disease with advanced fibrosis and cirrhosis. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements
Please look for future editions in which multiple aspects of our development programs for unmet medical needs will be addressed.
Reference List
1. Banerjee R, Pavlides M, Tunnicliffe EM, Piechnik SK, Sarania N, Philips R, et al. Multiparametric magnetic resonance for the non-invasive diagnosis of liver disease. J Hepatol 2014 Jan;60(1):69-77.
2. Singh S, Venkatesh SK, Loomba R, Wang Z, Sirlin C, Chen J, et al. Magnetic resonance elastography for staging liver fibrosis in non-alcoholic fatty liver disease: a diagnostic accuracy systematic review and individual participant data pooled analysis. Eur Radiol 2015 Aug 28.
3. Singh S, Venkatesh SK, Wang Z, Miller FH, Motosugi U, Low RN, et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: a systematic review and meta-analysis of individual participant data. Clin Gastroenterol Hepatol 2015 Mar;13(3):440-451.
4. Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le BB, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010 Feb;51(2):454-462.
Make a Comment or Ask a Question
[contact-form-7]The post Second Phase 2 clinical trial initiated for fatty liver disease with advanced fibrosis appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Lead product candidate GR-MD-02 shows no unfavorable drug-drug interactions appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>An important question in drug development, and the subject of U.S. Food and Drug Administration (FDA) regulations, is whether a drug candidate interacts with other medications the target patient population may also be taking. This is a critical issue, because many patients with chronic diseases are on multiple medications over long periods of time and may take other medications on an intermittent basis.
The basis of many drug-drug interactions is that the experimental new drug may use some of the same metabolic pathways that other drugs use. If this is the case, the new drug may inadvertently increase the levels of other drugs in the system, and thus alter their effect and increase their side effects. Prescribing physicians need to know about potential unfavorable interactions, if any, between an experimental new drug like GR-MD-02 and other drugs their patients may require.
In evaluating potential drug interactions, the first experiments with GR-MD-02 were done in test tubes and with cell cultures. Many metabolic pathways were evaluated in this way to determine whether there were potential interactions with GR-MD-02. Generally, the results of these experiments showed there was little to no risk of drug metabolism interactions with GR-MD-02, but the analysis of one particular metabolic enzyme, called CYP3A4, suggested there was small risk for interaction with other drugs. Therefore, the company agreed with the FDA to evaluate the possible interaction in humans of GR-MD-02 with a model drug for the CYP3A4 enzyme. The model drug used was midazolam, which is known as Versed® and is widely used for mild, conscious sedation.
We designed, conducted, completed, disclosed publicly (see our press release dated May 14, 2015) and reported to the FDA results of a Phase 1 study in normal healthy volunteer subjects. This study first tested drug levels of midazolam after a single intravenous (IV) dose, which served as a control. Midazolam was then administered again and drug levels were monitored following a single IV dose of GR-MD-02 (8 mg/kg) and following three weekly IV doses of GR-MD-02 (also 8 mg/kg). A total of 17 subjects completed the study, and all met the primary endpoint of no difference between midazolam levels when administered alone and in combination with single and multiple doses of GR-MD-02. Of note, 8 mg/kg of body weight of GR-MD-02 is the same dose we are testing in our current Phase 2 program in nonalcoholic steatohepatitis (NASH) patients with cirrhosis and with advanced fibrosis.
These Phase 1 results show that GR-MD-02 has no effect on the metabolism and serum levels of midazolam, and these results can be imputed to other drugs metabolized by CYP3A4 that are in common use. In fact, the CYP3A4 enzyme metabolizes about half of all the drugs currently on the market, according to published estimates. (Click here for more information on CYP3A4.) With the successful completion of this study, the company does not anticipate further drug-drug interaction studies will be required.
So, what does this science mean for the development of GR-MD-02? First, another 17 healthy individuals received up to three doses of GR-MD-02 without any significant adverse events, confirming the safety of the drug as seen in the first Phase 1 trial. Second, these findings allow patients on concomitant medications to be enrolled in our Phase 2 clinical trials with minimal concern for drug interactions, thus increasing the pool of potential patients that can be included in the trials. Finally, should GR-MD-02 receive marketing approval, patients and physicians will be less concerned our drug will interfere with other drugs they may be taking and we will not be faced with restrictive labeling regarding concomitant drug therapy. Therefore, successful completion of this drug interaction study in people checks another box in describing the underlying properties to support approval of GR-MD-02.
These “CEO Perspectives” will be a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “could,” “expect” and others. These statements include those regarding the hope that Galectin’s development program for GR-MD-02 will lead to the first therapy for the treatment of fatty liver disease with advanced fibrosis and cirrhosis. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements
Please look for future editions in which multiple aspects of our development programs for unmet medical needs will be addressed.
Make a Comment or Ask a Question
[contact-form-7]
The post Lead product candidate GR-MD-02 shows no unfavorable drug-drug interactions appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Welcome to CEO Perspectives appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>During the normal course of conducting business in a public company like Galectin Therapeutics, as the CEO and Chief Medical Officer of the company, I have conversations and interactions with many different firms and individuals on a daily basis. I am frequently discussing and fielding questions on science, our drug development programs, and many other topics from investors, analysts, scientists, physicians and others. At all times, the factual information that is discussed during these conversations has been previously publically disclosed and is available in our filings with the U.S. Securities and Exchange Commission. Everyone has access to all the information that I, or any other employee of the Company, discloses to individuals outside of the company.
In a highly technical area such as drug development, our investors and other interested members of the public have a great thirst for information and explanation of our programs I would like to help investors and members of the public understand our programs and answer questions that I routinely hear using a new communication medium that we designed, called “CEO Perspectives”. In this series of “CEO Perspectives” I will address key areas of activities for Galectin informed by questions that I receive from various constituents. It is my hope that these articles will help collate and clarify the already publically available information. Following each “CEO Perspective” there will be a comment section. Please use this to communicate your thoughts and questions. I hope you enjoy this new communication series and find it useful.
These “CEO Perspectives” will be a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may”, “could”, “expect” and others. These statements include those regarding the hope that Galectin’s development program for GR-MD-02 will lead to the first therapy for the treatment of fatty liver disease with advanced fibrosis and cirrhosis. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements
Please look for future editions in which multiple aspects of our development programs for unmet medical needs will be addressed.
Make a Comment or Ask a Question
[contact-form-7]The post Welcome to CEO Perspectives appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>