The post Pulmonary Arterial Hypertension is Associated with Elevated Levels of Galectin-3 and a Potential Therapeutic Target for GR-MD-02 appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>Galectin Therapeutics is collaborating with a number of academic groups to evaluate cardiovascular diseases that might be amenable to treatment with our galectin inhibitor drugs. In this article, I discuss the results obtained by the outstanding investigators at the Vascular Biology Center and the Department of Pharmacology and Toxicology at Augusta University (formerly known as Georgia Regents University), which were recently presented at the 2016 American Thoracic Society International Conference and highlighted in a recent press article.
What is pulmonary arterial hypertension (PAH)?
Pulmonary hypertension (PH) is high blood pressure in the main arteries that supply blood to the lungs, caused by the narrowing and constriction of blood vessels in the lungs themselves. This elevated blood pressure increases the work required of the heart’s right ventricle to pump blood into the lung, which eventually culminates in failure of the right ventricle, circulatory collapse and death. The causes of PH have been categorized into five groups. Group 1, also called pulmonary arterial hypertension (PAH), includes idiopathic and hereditary PAH, as well as multiple disorders that affect the small arterioles (blood vessels) in the lung. The remaining four groups are secondary to other disease including left heart failure (Group 2), chronic lung disease (Group 3), chronic pulmonary embolism (Group 4) or multifactorial mechanisms (Group 5).
The figure below on the left shows a normal pulmonary artery with the various layers of cells in the wall, including the external elastic lamina on the outside of the artery, the smooth muscle cell layer, the internal elastic lamina and the endothelial cell lining on the inside. As depicted in the figure below on the right, in PAH there is a marked increase in the thickness of the smooth muscle cell layer as well as fibrosis. This narrows the lumen of the artery and restricts blood flow.
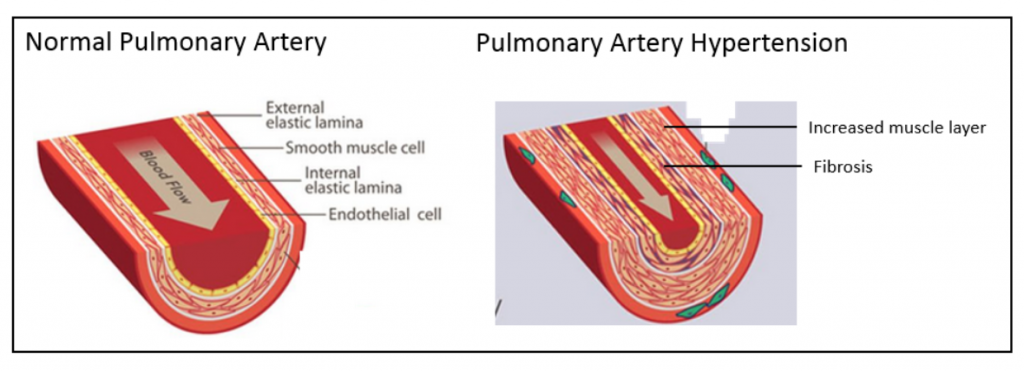
One of the more pressing needs is for new PH therapies in Group 1 disorders, particularly idiopathic PAH. Although there are a number of vasodilator drugs that are approved for the treatment of PAH, additional therapies are needed to address the structural narrowing of pulmonary arteries..
Galectin-3 protein is markedly elevated in human and experimental pulmonary arterial hypertension
The investigators at Augusta University experimented with three animal models of PAH using chemical treatments and/or low oxygen environments. These models are well established to cause narrowing of the pulmonary blood vessels leading to stress and failure of the right ventricle, meaning they mimic what happens in human PAH. The investigators found that the galectin-3 protein was markedly higher in the pulmonary arteries of diseased animals and the degree of increased blood pressure correlated with the level of galectin-3 elevation.
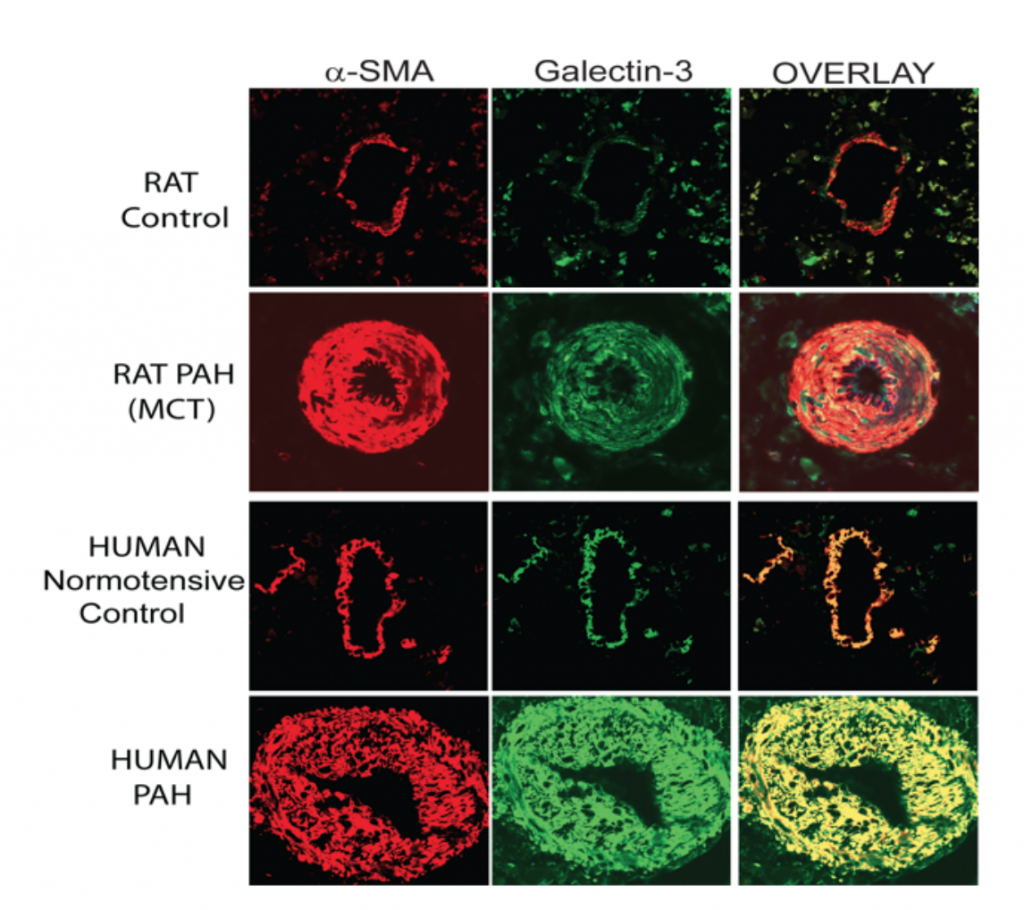
Since a picture is worth a thousand words, I have reproduced below images of the microscopic assessment of galectin-3 in pulmonary arteries in one rat model with monocrotaline treatment (MCT), as compared with the human disease.
The red stain is for the protein alpha smooth muscle actin (α-SMA), which stains smooth muscle cells in the artery, and the green stain is for the galectin-3 protein; the overlay combines the two images and shows that galectin-3 is predominantly in smooth muscle cells. In both the normal rat and the human pulmonary arteries, a thin layer of smooth muscle cells that contain galectin-3 is visible. In the rats and humans with PAH there is a marked increase in thickness of the muscle cells that surround the artery associated with a marked increase in galectin-3.
GR-MD-02 is effective in experimental pulmonary hypertension
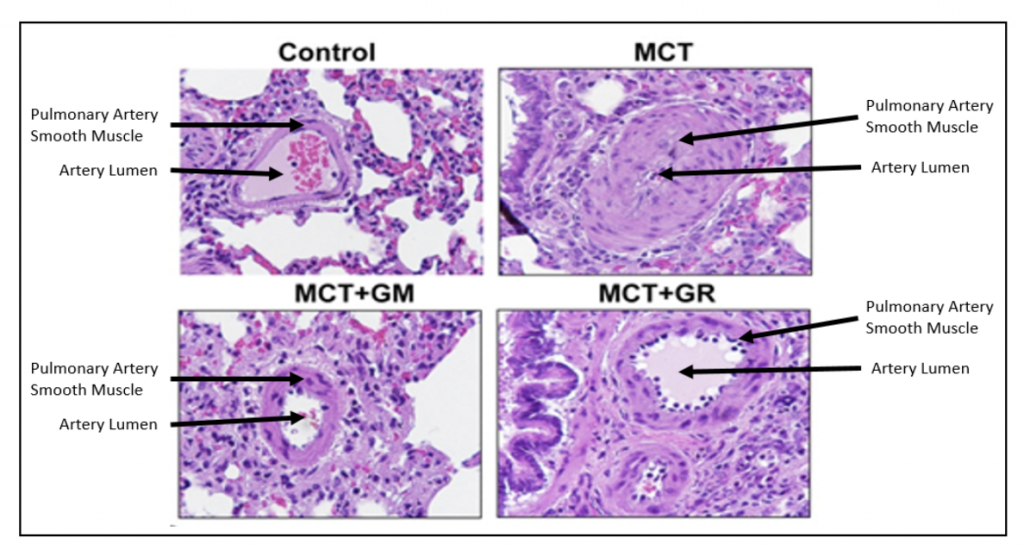
The investigators next tested whether our galectin-3 inhibitors were able to change the course of experimental PAH using the model in which the rats were given a single injection of MCT to induce pulmonary hypertension. Two of our galectin inhibitors were used as treatments: GR-MD-02, which is currently in clinical trials for NASH, psoriasis, and cancer, and GM-CT-01.
As shown in the figure below, the control (normal) rat pulmonary artery has a thin layer of smooth muscle cells surrounding it, whereas the animals treated with MCT have a markedly thickened smooth muscle layer that nearly blocks the lumen of the artery. Treatment of MCT animals with either GR-MD-02 or GM-CT-01 resulted in significantly fewer smooth muscle cells and a much larger artery lumen.
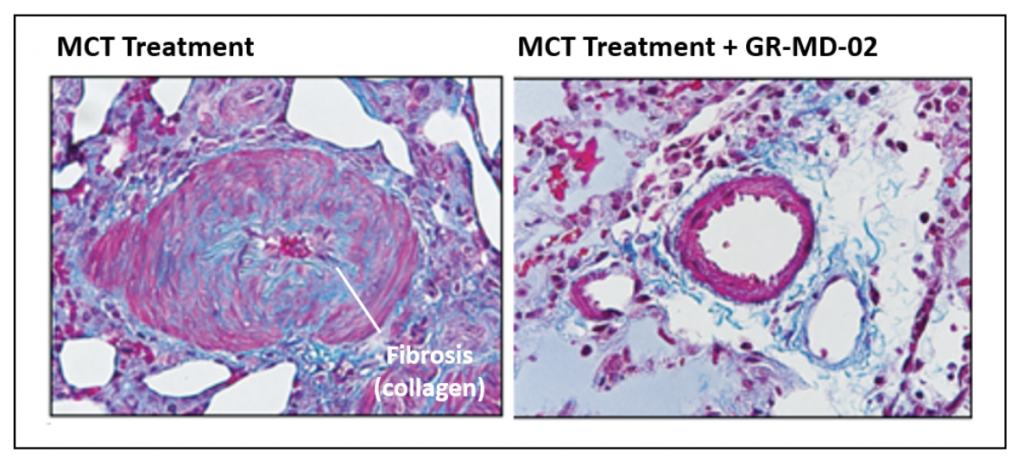
Below are images of pulmonary arteries stained to show fibrosis, mostly the protein collagen, which is identified by the light blue material. In the MCT treated animals, there is a significant amount of fibrosis associated with the smooth muscle cells in the pulmonary artery. This fibrosis is virtually eliminated in the artery wall following treatment with GR-MD-02, which provides a link to data showing improvement in fibrosis in other models of disease in liver, lung and kidney.
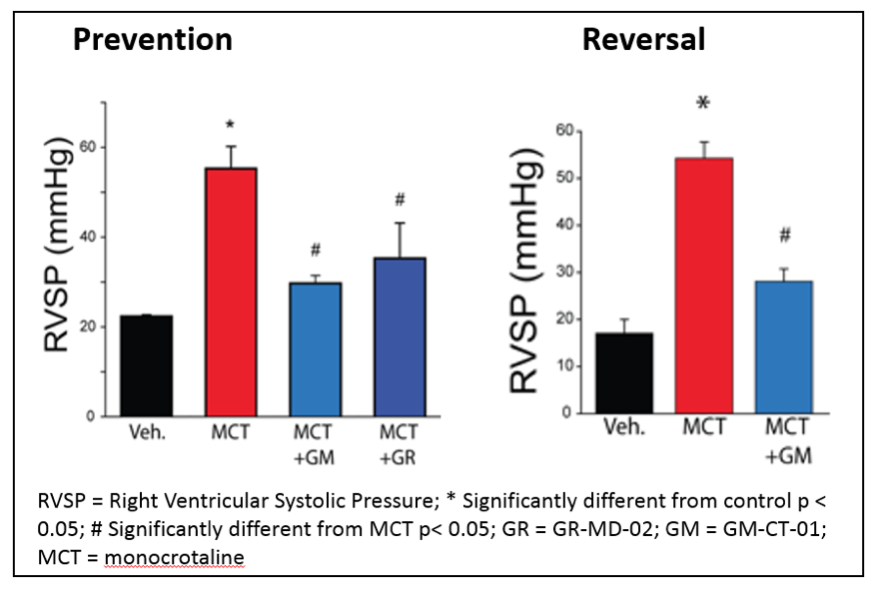
Treatment with either GR-MD-02 or GM-CT-01 also resulted in functional improvement in the PAH rats as indicated by reduced right ventricular systolic pressure (RVSP), as shown below. This improvement was seen whether the treatment was started immediately after the MCT injection (Prevention) or three weeks after the MCT injection (Reversal).
Prospects for human therapy
The results from these animal studies suggest that exploration of anti-galectin therapies, and specifically with GR-MD-02, might be a new therapeutic approach for these seriously ill patients. Currently there are approved therapies that help dilate constricted pulmonary arteries, but there are no therapies that effectively change the structure of the arteries or reverse the long-term course of the disease. A drug able to reverse the arterial smooth muscle and fibrosis findings in PAH might play an important role in treating these patients.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including whether GR-MD-02 and GM-CT-01 may be effective in the treatment of pulmonary arterial hypertension. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2015, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Make a Comment or Ask a Question
[contact-form-7]The post Pulmonary Arterial Hypertension is Associated with Elevated Levels of Galectin-3 and a Potential Therapeutic Target for GR-MD-02 appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post The Potential for Treatment of Liver Fibrosis With Galectin’s Drug Candidate GR-MD-02 appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>Liver fibrosis is scar formation in the liver which, when it reaches a large amount of scar tissue (called cirrhosis), results in liver failure and complications leading to death. The only available treatment for liver cirrhosis is a transplant, which is not only expensive, but is limited in supply (just over 6,000 annually in U.S.). Many liver diseases lead to fibrosis, including hepatitis, alcohol abuse, and fatty liver disease, which is called NASH (non-alcoholic steatohepatitis). Galectin is focused on liver fibrosis due to NASH because it is the most common liver disease and there are currently no available therapies.
Fatty liver disease, or NASH, can take decades to progress to advanced fibrosis and cirrhosis and we cannot predict which patients with early NASH disease will progress to advanced fibrosis. Galectin is encouraged by its galectin inhibitor, GR-MD-02, because it both reduces the inflammation of NASH and, importantly, can reverse liver fibrosis in pre-clinical animal models. The evidence for this has been published in peer-reviewed scientific articles (1, 2). Many companies in the NASH space are focused on early disease because the mechanism of their drug likely does not have a direct effect on fibrosis. In our opinion, our drug has a broader potential particularly in late stage NASH because GR-MD-02 has, in pre-clinical studies as reported in the above peer-reviewed articles, reversed advanced fibrosis and cirrhosis. In addition to not knowing which patients will progress to advanced fibrosis, a drug targeting early stage NASH is likely to require chronic administration for many years, thus possibly treating some patients unnecessarily, along with taxing the healthcare system with increased costs.
The medical community has encouraged our continued research as evidenced by references to our therapy in scientific publications. For example, Galectin Therapeutics’ compound that targets galectin-3 protein, GR-MD-02, is discussed in Expert Reviews in Gastroenterology and Hepatology, “In search of the magic bullet: can liver inflammation and fibrosis be reversed with medication?” (3) In their review of several potential mechanisms, the authors state that galectin-3 is “emerging as a possible target to revert liver fibrosis” and cite Galectin Therapeutics’ peer reviewed publication on preclinical data showing reversal of cirrhosis in an animal model and phase 1 clinical trial results.
Another recent reference is found in a review in Clinics and Research in Hepatology and Gastroenterology entitled “Novel therapeutic targets for steatohepatitis (4).” Arun Sanyal, M.D., of the Virginia Commonwealth University School of Medicine states, “Galectin has been targeted for the treatment of NASH with advanced fibrosis…Preliminary studies have confirmed its safety and tolerability. Phase 2 studies to clarify its efficacy are now under way.”
We are pleased that experts in the field have recognized our drug compound, GR-MD-02, as having potential in both the inflammation of NASH and in reversing advanced fibrosis. Galectin has initiated two phase 2 clinical trials to evaluate the possible reversal of liver fibrosis in NASH, the most common form of liver disease. The NASH-CX trial will focus on reversal of fibrosis in the most severe form of fibrosis called cirrhosis and the NASH-FX trial will attempt to show reversal of advanced fibrosis in a pre-cirrhotic stage.
These “CEO Perspectives” will be a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may”, “could”, “expect” and others. These statements include those regarding the hope that Galectin’s development program for GR-MD-02 will lead to the first therapy for the treatment of fatty liver disease with advanced fibrosis and cirrhosis. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements
Please look for future editions in which multiple aspects of our development programs for unmet medical needs will be addressed.
Reference List
- Traber PG, Chou H, Zomer E, Hong F, Klyosov A, Fiel MI, et al. Regression of fibrosis and reversal of cirrhosis in rats by galectin inhibitors in thioacetamide-induced liver disease. PLoS One 2013;8(10):e75361.
- Traber PG, Zomer E. Therapy of experimental NASH and fibrosis with galectin inhibitors. PLoS One 2013;8(12):e83481.
- Zimmerman HW, Tacke F. In search of the magic bullet: can liver inflammation and fibrosis be reversed with medications? Expert Rev Gastroenterol Hepatol 2015;1-3.
- Sanyal AJ. Novel therapeutic targets for steatohepatitis. Clin Res Hepatol Gastroenterol 2015 Jul 6.
Make a Comment or Ask a Question
[contact-form-7]The post The Potential for Treatment of Liver Fibrosis With Galectin’s Drug Candidate GR-MD-02 appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Welcome to CEO Perspectives appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>During the normal course of conducting business in a public company like Galectin Therapeutics, as the CEO and Chief Medical Officer of the company, I have conversations and interactions with many different firms and individuals on a daily basis. I am frequently discussing and fielding questions on science, our drug development programs, and many other topics from investors, analysts, scientists, physicians and others. At all times, the factual information that is discussed during these conversations has been previously publically disclosed and is available in our filings with the U.S. Securities and Exchange Commission. Everyone has access to all the information that I, or any other employee of the Company, discloses to individuals outside of the company.
In a highly technical area such as drug development, our investors and other interested members of the public have a great thirst for information and explanation of our programs I would like to help investors and members of the public understand our programs and answer questions that I routinely hear using a new communication medium that we designed, called “CEO Perspectives”. In this series of “CEO Perspectives” I will address key areas of activities for Galectin informed by questions that I receive from various constituents. It is my hope that these articles will help collate and clarify the already publically available information. Following each “CEO Perspective” there will be a comment section. Please use this to communicate your thoughts and questions. I hope you enjoy this new communication series and find it useful.
These “CEO Perspectives” will be a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may”, “could”, “expect” and others. These statements include those regarding the hope that Galectin’s development program for GR-MD-02 will lead to the first therapy for the treatment of fatty liver disease with advanced fibrosis and cirrhosis. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements
Please look for future editions in which multiple aspects of our development programs for unmet medical needs will be addressed.
Make a Comment or Ask a Question
[contact-form-7]The post Welcome to CEO Perspectives appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>