The post Fatty Liver is an Enormous Global Problem appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>Younossi and his colleagues collected data on over 8.5 million people from 22 countries, using a method called meta-analysis which allows combining data from multiple studies. (I have described this method in a previous Perspective.) Rinella and Charlton nicely summarized the main findings of this very important study in their editorial.
Before I describe the main findings, I must define some terms. Non-alcoholic fatty liver disease, or NAFLD, refers to abnormal accumulation of fat in liver cells. In some individuals, NAFLD progresses to NASH, or non-alcoholic steatohepatitis. In NASH, the presence of inflammation and dying liver cells is added to the accumulation of fat in liver cells. Some individuals with NASH have progressive fibrosis, or scarring of the liver, which may ultimately lead to cirrhosis, complications of cirrhosis, the need for liver transplant, and death.
The first conclusion of the study is that approximately one in four people in the world are affected by NAFLD. There are geographical differences in the rates of NAFLD across the world, as shown in the figure below published in the editorial (the numbers represent the percent of people in that region who have NAFLD).
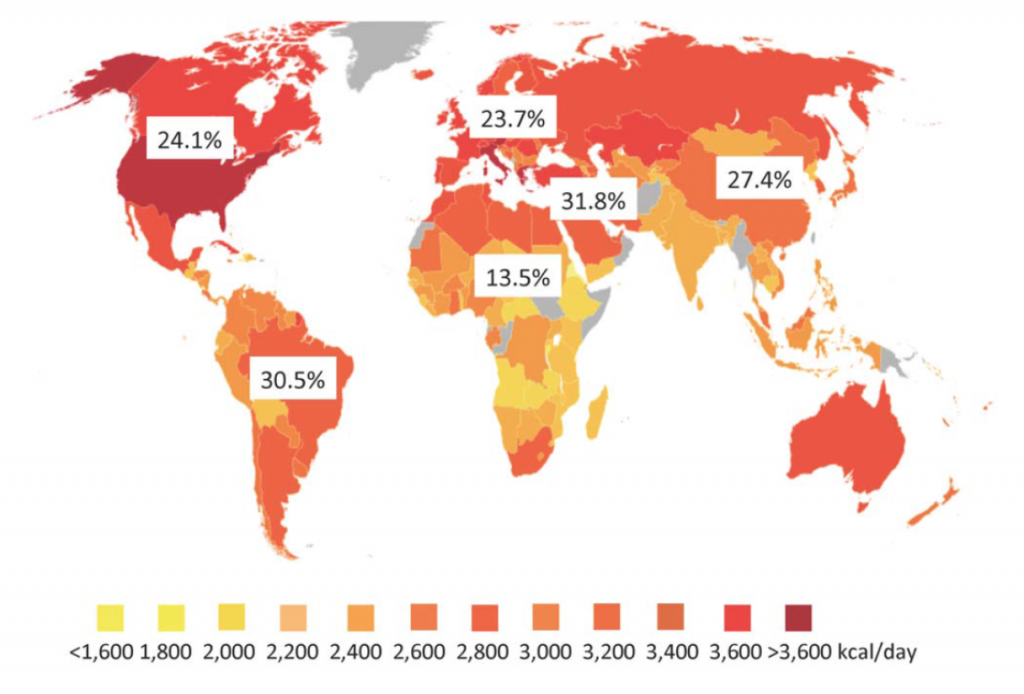
The color coding on this graph relates to the average caloric intake in the individual countries. While it is well known that obesity is associated with NAFLD, the relatively poor correlation with caloric intake with NAFLD rates suggests there are other factors involved. Some of these factors may include genetics (important in some regions), the type of food ingested, the composition of bacteria in the gut, and others.
The second major point taken from this study is that progression of fibrosis in NAFLD is slow and unusual. The editorial authors include an instructive figure to demonstrate this point. Out of 100 patients with NAFLD, only 5 will develop cirrhosis (the end stage of scarring) and only 1-2 patients will die as a result of that cirrhosis or require a liver transplant.
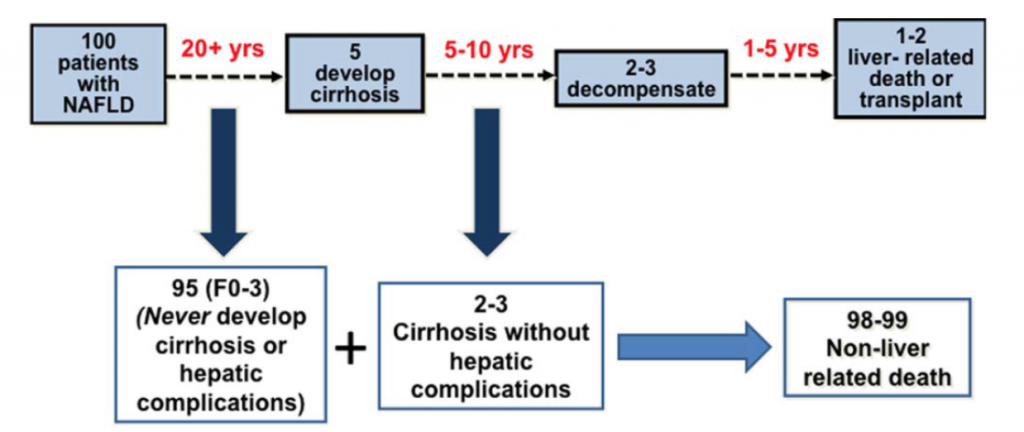
On face value, this seems like a small number of patients dying of their liver disease. However, when one considers the large number of people with NAFLD, those with cirrhosis represent a very significant problem. As stated by the authors, “Despite the low frequency of liver related mortality, the staggering denominator of over 1 billion adults with NAFLD coupled with a life-time risk of ~2% for liver-related mortality will eventually translate into ~20,000,000 liver-related deaths among patients currently alive with NAFLD.”
The final point is that NAFLD is, or will soon become, the most common cause of liver disease and liver-related death globally. The most important target for dealing with this enormous health problem is related to life-style changes to maintain a healthy liver, as I described in a previous Perspective. However, since there are currently no medicines available for this disease, we must also focus on those 20,000,000 people who are destined to die of NASH cirrhosis. This is the target for our Phase 2 clinical trial, NASH-CX, using our drug candidate GR-MD-02.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. For a discussion of factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2015, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements.
Reference List
1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016 Jul;64(1):73-84.
2. Rinella M, Charlton M. The globalization of nonalcoholic fatty liver disease: Prevalence and impact on world health. Hepatology 2016 Jul;64(1):19-22.
Make a Comment or Ask a Question
[contact-form-7]The post Fatty Liver is an Enormous Global Problem appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Why Fibrosis is a Hot Area for Pharmaceutical Research appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>Bristol-Myers Squibb ($BMS) recently paid $150 million for the exclusive right to acquire Promedior, a clinical-stage biotechnology company in the midst of a Phase 2 trial on the treatment of idiopathic pulmonary fibrosis (IPF) and myelofibrosis (MF). If clinical trials are successful, the deal could be worth as much as $1.25 billion. This is just one of a number of fibrosis-related acquisitions for BMS over the past few years, and BMS is not the only company active in this market. Fibrosis is a hot area right now. But why so?
The scientific community has known for a long time that fibrosis is a common final pathway for many different diseases. An investigator at the NIH suggested in 2004 that as many as 45 percent of the deaths in the United States are related to fibrosis (1). That would include, in addition to liver fibrosis, heart, kidney, lung, skin, and arterial fibrosis – all organs that can be scarred as the result of a chronic disease, which then leads to organ failure and death.
While we can certainly work on curing each individual chronic disease that causes fibrosis, another approach would be to inhibit the fibrotic process. This approach could potentially be applied to a lot of other diseases as well — it’s been something of a Holy Grail in medicine for decades.
It’s a great idea. Unfortunately, there hasn’t been very much progress in the development of anti-fibrotic agents, until now.
Recently, there has been promising work in agents that appear to inhibit fibrosis — for example, our own GR-MD-02, which in our preclinical studies has shown to not only prevent fibrotic damage, but even reverse it. I think these breakthroughs are happening now because the medical community is coming to understand that fibrotic diseases, such as NASH, are complex conditions that require agents that affect multiple pathways in the fibrogenic process.
Let me give an example. There are many important cytokines in the inflammatory process, so it might make sense to inhibit one of them and see how that changes the inflammatory or the fibrotic process. However, by doing that, you are ignoring dozens of other cytokines that are also involved in the inflammatory process. Even if you inhibit the one, you’re not having any impact on the other cytokines involved.
Therefore, agents that address a number of different cell types in the liver that express a wide range of cytokines and fibrotic mediators may be more effective than a pinpoint attack on a particular cytokine or mediator. GR-MD-02 appears to affect macrophages as well as activated myofibroblasts in the liver, which inhibits a wide range of different fibrotic processes. It may be that affecting master regulatory cells involved in inflammation and fibrosis is going to be more effective than affecting any individual mediators.
But as I pointed out, fibrosis is a problem in many other organs than the liver. My sense of it is, if you’re going to have a drug that is robust for a particular organ fibrosis, it’s more than likely going to work for other fibrotic diseases. That’s one of the reasons why we have done pre-clinical studies of GR-MD-02 in lung, kidney and heart, and it’s shown an effect in those as well. That is also, I suspect, part of BMS’ calculations in acquiring Promedior. IPF and lung fibrosis is an indication that creates a large enough market on its own, but the potential to address a disease process involved in 45 percent of the deaths in the U.S. can be found in the background of any fibrosis drug.
That is one of the things that makes GR-MD-02 different than many of the other drugs being developed to address NASH and fatty liver disease. Most of the dozen or so compounds don’t address the core fibrotic process, so even if they work in NASH, they’re not likely to work elsewhere in the body. Only the truly anti-fibrotic drugs like GR-MD-02 have the potential to address fibrosis in other organs.
These “CEO Perspectives” will be a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may”, “could”, “expect” and others. These statements include those regarding the hope that Galectin’s development program for GR-MD-02 will lead to the first therapy for the treatment of fatty liver disease with advanced fibrosis and cirrhosis. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements
Please look for future editions in which multiple aspects of our development programs for unmet medical needs will be addressed.
Reference List
- Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol 2004 Aug;4(8):583-594.
Make a Comment or Ask a Question
[contact-form-7]The post Why Fibrosis is a Hot Area for Pharmaceutical Research appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Lessons Learned from the Failure of Raptors’ Phase 2b NASH Trial appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>On September 14, 2015, Raptor Pharmaceutical Corp. (NASDAQ: RPTP) announced top-line results of its Phase 2b trial evaluating RP103 in children with biopsy-confirmed nonalcoholic steatohepatitis (NASH). As reported here, the study failed to meet its primary endpoint of a two-point decrease in a fatty-liver-disease scoring system and no worsening of fibrosis, or scarring of the liver. It’s always disappointing when a promising drug candidate fails in a clinical trial, especially when it addresses something like NASH, for which there really aren’t any proven treatment options.
The failure of the Raptor trial provides an important lesson for any company developing therapies to address fatty liver disease and NASH on the use of surrogate blood markers in tracking the progression (or regression) of fatty liver disease and NASH.
If you’ve ever had routine blood work done as part of a physical, the tests probably included the measurement of the transaminases AST (aspartate aminotransferase) and ALT (alanine aminotransferase). These are enzymes that naturally circulate in the blood. They are also enzymes that are in cells and, in particular, they are in liver cells. If there is damage to the liver, as with a viral hepatitis A infection, these enzymes will be released into the blood serum in great numbers. A marked increase in the transaminases in your blood is one of the ways doctors diagnose hepatitis and other liver diseases.
Transaminases are often elevated in fatty liver disease and NASH. If you recruit a group of NASH patients, on average, the liver enzymes are going to be elevated. It’s easy to make the logical conclusion that if you have a drug that might work in NASH, and you treat those patients, the transaminase levels should come down. A number of trials have been undertaken with that presumption, and one of them was Raptor’s.
Between 2008 and 2010, Raptor conducted a small, open-label trial (meaning there wasn’t a placebo arm in the trial) with its drug – a long-acting, slow-release cysteamine – in adolescents with NASH (mean age 14). Eleven patients completed the trial and seven patients had either normalization or >50% reduction of their transaminase (ALT) level from baseline. These results led the investigators to suspect the drug was having activity in NASH. They quickly moved on to the latest Phase 2b study; Raptor even received the support of the National Institutes of Health (NIH), which agreed to conduct and help fund the trial.
In this newest study, the transaminase levels of the patients indeed went down, but liver biopsies showed no change in the histology of that organ. This is evidence that the changes in transaminase levels seen in NASH patients are not necessarily indicative of the activity of the underlying disease. While there may be a correlation with some treatments, this trial shows that this is not always the case. In reality, patients with fatty liver disease and NASH will see their levels of transaminases fluctuate widely. Roughly two-thirds of all patients, at any given time, will have normal transaminases. There’s no good evidence that transaminases correlate with either the stage or the activity of the disease.
This failure of the Raptor study also has a broad implication for all clinical trials involving NASH because it demonstrates that serum transaminases cannot be relied upon as an early biomarker of the effectiveness of a drug. It may also have ramifications for other potential biomarkers. The lesson from Raptor’s failure shows that we need to be skeptical about serum biomarkers until they’re well correlated with changes in the liver itself.
Here at Galectin we are ever mindful of the need to correlate biomarkers with disease and how difficult the task is. In our Phase 2 program in NASH with advanced fibrosis and cirrhosis, we are performing multiple tests to ensure we have correlation with the underlying disease. In the NASH-CX trial we are performing both invasive (including biopsy) and non-invasive physical and metabolic tests to evaluate correlation (see discussion here) and the NASH-FX trials utilizes three of the most promising methods for non-invasive imaging methods to assess physical properties of the liver that correlate with fibrosis (see discussion here).
While not every clinical trial ends with success, they all help advance our understanding of fatty liver disease and NASH. It’s just a matter of learning the lessons they teach us.
These “CEO Perspectives” will be a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “could,” “expect” and others. These statements include those regarding the hope that Galectin’s development program for GR-MD-02 will lead to an additional therapy for the treatment of psoriasis. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements
Please look for future editions in which multiple aspects of our development programs for unmet medical needs will be addressed.
Make a Comment or Ask a Question
[contact-form-7]The post Lessons Learned from the Failure of Raptors’ Phase 2b NASH Trial appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Clinical Trial to Establish Efficacy of GR-MD-02 in NASH Cirrhosis appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>In late June 2015, Galectin Therapeutics initiated a Phase 2 clinical trial (the NASH-CX trial) to evaluate the ability of its anti-galectin drug GR-MD-02 to treat patients with cirrhosis of the liver due to fatty liver disease (non-alcoholic steatohepatitis, or NASH). Cirrhosis is advanced-stage scarring, or fibrosis, in which the liver is “clogged” with fibrous tissue that distorts the organ’s architecture and results in increased resistance to blood flow to the liver and dysfunction of liver cells. The goal of therapy with GR-MD-02 is to reduce fibrosis and, in turn, improve liver function and positively affect patient outcomes.
One of the most important aspects of any clinical trial is the “endpoint” that will be measured as evidence of the drug’s effect. Endpoints include the “primary” endpoint on which the overall success of the trial is based, and “secondary” endpoints that are supportive of the primary endpoint. The strongest endpoints for any clinical trial are those related to patient outcomes which include how a patient feels, functions, or survives (mortality). However, these endpoints in chronic diseases often can only be measured in the end stage of disease, which often occurs many years after the patient contracts the disease. Therefore, many clinical trials are conducted with surrogate endpoints that are linked to patient outcomes. For drugs such as GR-MD-02 that fulfill a significant unmet need, the U.S. Food and Drug administration (FDA) may allow the initial approval of drugs based on achieving a reasonable surrogate endpoint under an accelerated approval pathway.
For NASH cirrhosis, potential surrogate endpoints have been proposed by experts in the field in joint discussions with the FDA (1) including measurement of change in hepatic venous pressure gradient (HVPG), which is a measure of pressure in the main blood supply to the liver (viz., portal pressure). In the NASH-CX trial, we are using a reduction in HVPG as the primary endpoint for evaluating efficacy. Secondary endpoints in the study, some of which are also potential surrogate endpoints that have been discussed with the FDA, include liver biopsy evaluations of fibrosis, non-invasive diagnostic tests including FibroScan® to measure liver stiffness, which correlates with fibrosis, and the 13C-methacetin breath test (MBT, Exalenz Bioscience Ltd.) (2), which is a measure of liver metabolic function. Further secondary endpoints also include direct patient outcome measures related to complications of cirrhosis. We believe, based on our discussions with the FDA, that these endpoints will provide a broad and relevant set of measures to determine whether GR-MD-02 could be an effective therapy for NASH cirrhosis.
While a clinical trial protocol such as the NASH-CX trial is a detailed and complicated document (nearly 250 pages long in our case), the fundamentals are straightforward, as shown in the figure below. All patients enrolled do have cirrhosis and portal hypertension, but are well-compensated, meaning that they have not yet had complications related to their cirrhosis. Patients who meet the inclusion and exclusion criteria will be randomly allocated to receive either placebo or one of two doses of GR-MD-02. The trial is double-blinded, meaning neither the patients nor their physicians, nurses or Galectin Therapeutics will know what therapy they are receiving. The various diagnostic evaluations will be done as indicated in the figure and for each patient the changes in these evaluations at the end of treatment will be compared with the evaluations performed at the beginning of the study. The length of the study is related to the duration of GR-MD-02 treatment as well as the number of patients in the study to achieve a necessary sample size for scientific and statistical evaluations. For this study, a total of 156 patients will each receive either a dose of GR-MD-02 drug or placebo in biweekly infusions for one year.
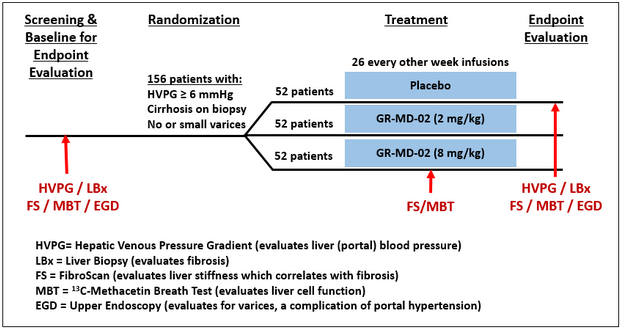
A number of investors have asked about the actual conduct of the clinical trial, so I will provide some details. We have engaged a large, multinational contract research organization (CRO) that is experienced in performing studies in liver disease, NASH, and cirrhosis. All study sites will be in the U.S. and will include a mix of large academic centers with internationally recognized clinical experts and prominent community-based health care centers. The two co-principal investigators for the trial, Dr. Naga Chalasani from Indiana University and Dr. Stephen Harrison from San Antonio Military Medical Center, are recognized experts in the field and were investigators in our Phase 1 clinical trial in NASH patients with advanced fibrosis.
Our most important operational milestone is the last patient randomized, in other words the first infusion of drug or placebo for patient number 156, which is targeted for August 2016. With a one-year treatment phase, if we meet this milestone then all patients will have completed therapy as of August 2017, with a goal for reporting top-line data by the end of 2017. Using the experience of our CRO in other trials of this type, we believe that we may achieve this goal with 45 study sites, but are able to increase that number to 60 study sites if necessary to stay on schedule. At this time, we have identified and pre-certified 55 study sites across the U.S., and are rapidly engaging to a total of 45 fully active study sites.
Fully establishing sites to enroll patients includes gaining approval from each of their investigational review boards (IRB), clinical and indemnification contracts, financial contracts, and education and training of their personnel. After sites are initiated, patients are screened in a process that involves performing multiple laboratory tests at different times, various invasive tests such as an upper endoscopy (EGD), liver biopsy and HVPG, and non-invasive tests such as FibroScan and MBT; this testing alone may take up to eight weeks. Assuming a patient passes the various screening parameters for entry into the trial from all these tests, they are then randomized and begin receiving bi-weekly infusions of drug or placebo. Sites are currently screening, enrolling and treating patients.
What do we expect to learn from this study? First, we expect to learn whether GR-MD-02 can reduce the portal pressure in a patient population of well-compensated cirrhosis, a measure that is known to be directly related to complications of cirrhosis and patient mortality. Second, we expect to learn whether our drug reduces portal pressure by reducing the amount of scarring in the liver as determined by liver biopsy. Finally, we anticipate learning how a reduction in fibrosis and portal pressure correlates with non-invasive testing such as FibroScan, MBT, serum biomarkers and other tests including a quality-of-life questionnaire and complications of cirrhosis. The NASH-CX trial is a rigorously designed study to gain the most information possible regarding the effect of GR-MD-02 in these patients, who have no current available therapy. The NASH-CX trial also importantly serves to help design later-stage trials such as those that may be required for regulatory approval. With success in this trial, GR-MD-02 could be the first drug shown to reduce fibrosis in cirrhotic patients and the first hope of a treatment in this patient population other than liver transplant.
More information on Galectin’s NASH-CX trial can be found at clinicaltrials.gov
- NASH-CX: A Multicenter, Randomized, Placebo-controlled, Double-blind, Parallel-group, Phase 2 Clinical Trial to Evaluate the Safety and Efficacy of GR-MD-02 for the Treatment of Liver Fibrosis and Resultant Portal Hypertension in Patients With Nash Cirrhosis https://clinicaltrials.gov/ct2/show/NCT02462967?term=GR-MD-02&rank=3
These “CEO Perspectives” will be a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “could,” “expect” and others. These statements include those regarding the timing of enrollment of patients and the hope that Galectin’s development program for GR-MD-02 will lead to the first therapy for the treatment of fatty liver disease with advanced fibrosis and cirrhosis. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements
Reference List
1. Sanyal AJ, Friedman SL, McCullough AJ, Dimick-Santos L. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: findings and recommendations from an American Association for the Study of Liver Diseases-U.S. Food and Drug Administration Joint Workshop. Hepatology 2015 Apr;61(4):1392-1405.
2. Exalenz Bioscience Ltd. (Israel). See www.exalenz.com for information on Exalenz and the 13C-methacetin breath test
Make a Comment or Ask a Question
[contact-form-7]The post Clinical Trial to Establish Efficacy of GR-MD-02 in NASH Cirrhosis appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>