The post Two Clinical Trials Using GR-MD-02 Galectin-3 Inhibitor in Combination with Cancer Immunotherapy Drugs appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The galectin-3 protein appears to play an important role in the progression of many cancers, especially with respect to the ability of cancer cells to hide from the body’s immune response. With combination immunotherapies becoming increasingly important in the treatment of cancer, inhibiting galectin-3 could provide another way to boost the body’s ability to fight cancer.
While liver fibrosis remains the primary focus of Galectin Therapeutics, we are pleased to support the Providence Cancer Center in Portland, Oregon, in exploring the use of our GR-MD-02 galectin-3 inhibitor in combination with the commercial immunotherapeutic agents Keytruda® (pembrolizumab) and Yervoy® (ipilimumab) in patients with advanced melanoma. Should using GR-MD-02 prove effective against melanoma, it could be a component of therapy in the quest for cancer cures.
We recently announced that Providence Cancer Center filed an Investigational New Drug (IND) application with the FDA to study GR-MD-02 in combination with Keytruda in a Phase 1b study of patients with advanced refractory metastatic melanoma. This study joins an ongoing Phase 1b study of GR-MD-02 in combination with Yervoy in patients with malignant melanoma, which is also being conducted by researchers at Providence Cancer Center.
The IND filing was prompted by findings from preclinical studies led by William L. Redmond, Ph.D., of the Providence Cancer Center’s Earle A. Chiles Research Institute. Those studies found that GR-MD-02 increased tumor shrinkage and enhanced survival in immune-competent mice with multiple types of cancers when combined with checkpoint inhibitors such as Yervoy or Keytruda.
We could not have a better partner than the Providence Cancer Center and the group undertaking this research. This is a prominent team of investigators led by Walter J. Urba, M.D., Ph.D., who established the Center in 1993 after working at the National Cancer Institute. They have access to a very strong, large and innovative group of scientists and clinicians who are experts in immunotherapy. Not only were they one of the sites involved with Bristol-Myers Squibb’s (BMS) first trials with Yervoy, they’ve also developed one of their own monoclonal antibodies (anti-Ox40) that was out licensed to MedImmune via a spin-off company called AgonOx, and which is now in clinical trials.
The Providence Cancer Center performed the preclinical work with GR-MD-02 that supports the clinical work now underway. Based on the preclinical results, it was decided that the data showed a sufficiently robust effect to undertake these combination clinical trials. The Providence Cancer Center is paying for the studies, and we are providing them with GR-MD-02. More than anything, I believe the fact that one of the top research groups in the country is funding this research using GR-MD-02 reinforces the potential importance of our drug in cancer immunotherapy.
A Bit of Background on the Trials
The first patient in the combination GR-MD-02/Yervoy trial was dosed in July, 2014, when Yervoy was the only cancer immunotherapy drug on the market. This first trial was in melanoma because that’s the indication for which Yervoy is approved. While Yervoy, a CTLA-4 inhibitor, is truly a revolution in the treatment of melanoma, the long-term survival in patients with advanced melanoma is only about 10 to 15 percent. With upwards of 85 percent of patients dying of their disease while being treated with Yervoy monotherapy, there was clearly a big opportunity for improvement.
The Yervoy trial has enrolled two cohorts at two dose levels. This is a dose-escalation trial, so we started with a low dose of 1 mg/kg of GR-MD-02, progressing to 2 mg/kg, and so on. We just finished dosing the 2 mg/kg group and observed no adverse events related to GR-MD-02, so now the next dosing cohort is underway, which is 4 mg/kg. Once we have three patients at that level, we will then proceed to 8 mg/kg and enroll 10 patients in that high-dose group.
Results in the Yervoy trial are being evaluated by looking at the size of the tumor – meaning does it shrink or grow – and also by measuring multiple types of immune cells in circulation. This last approach may give us an early indication of whether GR-MD-02 is providing some effect. One of the benefits of working with the Providence Cancer Center is that they’re one of the leading groups working in this area.
We could dose higher than 8 mg/kg, but we’ll have to assess the results we see at that dosage to determine whether we go higher. For instance, if we see immune markers that increase a small amount at 2 mg/kg, even more at 4 and then they level off at 8, we might well think we’re getting the maximum effect. If we see that the markers are continuing to increase at 8, we might then move on to higher doses.
The Keytruda trial, which has been cleared by the FDA, will would follow much the same approach.
Keytruda, a PD-1 inhibitor, works on an entirely different biological pathway than Yervoy. Keytruda was approved by the FDA last year for use in melanoma, and it shows an even greater effect than Yervoy with a 20 to 25 percent long-term survival rate. My last CEO Perspective touched on how powerful it can be to use two immunotherapy agents in combination, in particular Yervoy and Opdivo, which is also a PD-1 inhibitor. I would expect Yervoy and Keytruda to yield similar results if they were ever to be used in combination.
For our part, we are excited that GR-MD-02 is entering a trial for use in combination with Keytruda. The preclinical science looks promising. Why are we using Keytruda rather than Opdivo? Frankly, Providence Cancer Center and Galectin Therapeutics chose Keytruda because it was approved and on the market at the time we started to develop these studies. Now that we are set to begin the studies, I would expect enrollment to be even faster than the Yervoy study, as Keytruda is a more efficacious drug and it’s indicated for people who have failed Yervoy.
These “CEO Perspectives” are a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “might,” “could,” “expect” and others. These statements include those regarding the hope that Galectin Therapeutic’ s development program for GR-MD-02 will show that it can be both safe and effective when used in combination with other drugs for the treatment of patients with cancer. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements
Please look for future editions in which multiple aspects of our development programs for unmet medical needs will be addressed.
Make a Comment or Ask a Question
[contact-form-7]The post Two Clinical Trials Using GR-MD-02 Galectin-3 Inhibitor in Combination with Cancer Immunotherapy Drugs appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Exploratory Indication in Psoriasis appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>Serendipity, or fortunate happenstance, has always been an important part of the search for medicines. As pointed out by Thomas A. Ban (1), serendipity in drug discovery is more than just luck, but rather is best described by Pasteur’s famous quote that “Chance favors the prepared mind”. Serendipity may have entered our development program with our anti-galectin drug, GR-MD-02, and today we kick off another Phase 2 clinical trial to test whether an unexpected effect observed in our Phase 1 trial is truly something of significance.
Our surprising clinical trial observation
In the course of our Phase 1 clinical trial of GR-MD-02 in patients with fatty liver disease (NASH) and advanced fibrosis, we encountered a patient with plaque psoriasis, who happened to be one of the patients treated with four infusions of 4 mg/kg of GR-MD-02. Psoriasis is a relatively common, chronic skin disorder that causes a red, thickened and scaling rash that can cover many parts of the body.
Upon the patient’s follow-up visit to her physician, she reported resolution of her long-standing psoriasis. In an interview with a dermatologist and myself, the patient confirmed that she had severe psoriasis involving much of both arms continuously for six years. Following treatment with GR-MD-02, the psoriasis resolved and has not recurred for over a year! There was another patient in the study with mild psoriasis who was able to reduce use of topical steroids and another with severe psoriasis who had just been withdrawn from therapy with Stelara (ustekinumab), a powerful injection therapy for psoriasis, who did not improve.
Observation prompts exploratory clinical trial
On the strength of the marked effect in the one patient, both a dermatologist investigator and we felt it was worth investigating whether this was a real effect of GR-MD-02. In addition to the clinical finding, research publications suggest the potential importance of galectin-3 in psoriasis (2, 3). Therefore, we initiated a phase 2a, open label clinical trial in patients with moderate to severe plaque psoriasis (press release September 22, 2015). In this trial, 10 psoriasis patients with ≥ 10% of their skin affected and a PASI (psoriasis activity and severity index) of ≥ 12 points will be treated with 7 every other week infusions of 8 mg/kg GR-MD-02. The severity of psoriasis was agreed upon with the FDA as warranting experimental therapy. More information on the trial can be found at: https://clinicaltrials.gov/ct2/show/NCT02407041?term=GR-MD-02&rank=5
The primary endpoint for evaluation of these patients will be the PASI-75, which means that they have had a 75% improvement in the severity of the disease 30 days following the final infusion. This is a standard endpoint used in studies of drugs that have been approved for marketing. The PASI system assesses each affected body area as to the extent of skin redness, thickness, and scaling. We intend on reporting data 30 days following treatment of the last patient treated. Additional results would then be reported following 6 month and 1 year follow up periods to assess for durability of response.
What is next following exploratory trial?
Our current trial is based on an observation with one patient. It is promising enough to investigate in an exploratory trial, but single-patient observations are notoriously unreliable. Therefore, while we anticipate results, we are cautious in our optimism.
We will consider this clinical trial successful if ≥ 50% of patients treated meet the primary efficacy endpoint. Positive results from this trial would be significant, since we would have demonstrated a potentially important clinical effect of our galectin-3 inhibiting drug, GR-MD-02. Moreover, it is of interest that there is a relatively high incidence of NASH in patients with psoriasis (4, 5), which is our other major indication for GR-MD-02.
With positive results, we will then consider entering into a development program aimed at marketing approval for psoriasis. There are already multiple excellent drugs on the market for this indication, some of which have been approved in the last few years. Potential areas that GR-MD-02 may be differentiated from other drugs include safety, prolonged response (if the one patient results are confirmed), and lower cost manufacture.
These “CEO Perspectives” will be a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “could,” “expect” and others. These statements include those regarding the hope that Galectin’s development program for GR-MD-02 will lead to the first therapy for the treatment of fatty liver disease with advanced fibrosis and cirrhosis. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements
Please look for future editions in which multiple aspects of our development programs for unmet medical needs will be addressed.
Reference List
1. Ban TA. The role of serendipity in drug discovery. Dialogues Clin Neurosci 2006;8(3):335-344.
2. Chen HY, Lo C-H, Li C-S, Hsu DK, Liu FT. Galectins and cutaneous immunity. Dermatologica Sinica 2012;30:121-127.
3. Lacina L, Plzakova Z, Smetana K, Jr., Stork J, Kaltner H, Andre S. Glycophenotype of psoriatic skin. Folia Biol (Praha) 2006;52(1-2):10-15.
4. Roberts KK, Cochet AE, Lamb PB, Brown PJ, Battafarano DF, Brunt EM, et al. The prevalence of NAFLD and NASH among patients with psoriasis in a tertiary care dermatology and rheumatology clinic. Aliment Pharmacol Ther 2015 Feb;41(3):293-300.
5. Wenk KS, Arrington KC, Ehrlich A. Psoriasis and non-alcoholic fatty liver disease. J Eur Acad Dermatol Venereol 2011 Apr;25(4):383-391.
Make a Comment or Ask a Question
[contact-form-7]The post Exploratory Indication in Psoriasis appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>The post Second Phase 2 clinical trial initiated for fatty liver disease with advanced fibrosis appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>As a company, we are focused on developing a therapy for patients with non-alcoholic steatohepatitis (NASH), or fatty liver disease, with advanced fibrosis (scarring) of the liver. Our drug GR-MD-02 has been shown to prevent and reverse fibrosis in preclinical animal models and there was evidence of a therapeutic effect in our Phase 1 trial (see previous CEO Perspectives). With the recent start of our second Phase 2 clinical trial (see September 16, 2015 press release), we now have studies underway that address two distinct NASH patient populations including those with cirrhosis, the most advanced stage of fibrosis, and those with advanced fibrosis without cirrhosis.
The newly announced NASH-FX clinical trial, discussed here, will attempt to show reversal of advanced fibrosis in a pre-cirrhotic stage. The goal is to reduce fibrosis to an early stage, slowing or preventing the patient from progressing to cirrhosis. Therefore, the NASH-FX trial addresses a very different patient population than the NASH-CX trial which treats NASH cirrhosis (see previous CEO Perspectives), which could lead to a separate indication.
The NASH-FX trial will treat NASH patients with advanced fibrosis with 8 mg/kg of GR-MD-02 or placebo for four months (see diagram below). The Principal Investigator, a prominent liver physician, and we believe that this is a sufficiently long period of time to show an effect on fibrosis given the results and treatment time of the Phase 1 trial. Additional trial details can be found at: https://clinicaltrials.gov/ct2/show/NCT02421094?term=GR-MD-02&rank=4
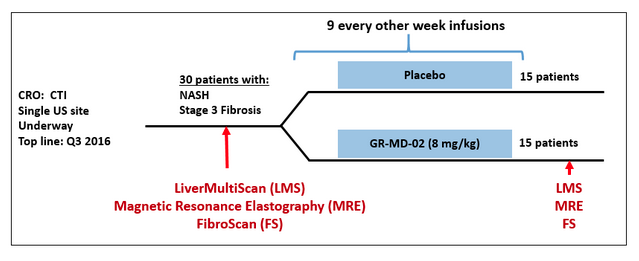
In the NASH-FX trial we will be using non-invasive imaging tests of the liver to assess fibrosis. The most definitive test for liver fibrosis involves microscopic evaluation of liver tissue obtained via a biopsy. The NASH-CX trial, for example, is using liver biopsy as one of the multiple evaluations of fibrosis in that trial. However, the short duration of the NASH-FX trial precludes repeated liver biopsies in patients because of safety concerns and potential discomfort related to the biopsies.
Liver biopsy is not a perfect test, as interpretation of liver biopsies is fraught with inconsistencies due to sampling error since the tissue piece obtained only represents approximately 1/50,000th of the liver. These inconsistencies along with the risk of the procedure itself have led investigators across the world to seek better, non-invasive approaches to evaluate liver fibrosis. One approach is to evaluate blood tests that may reflect the state of fibrosis in the liver, but this has not yet yielded results that are useful in clinical trials.
Better results have been obtained with methods to image the liver and assess physical characteristics of the liver tissue that is reflective of fibrosis. In the NASH-FX trial, we are using three of the most promising methods for directly assessing physical properties of the liver that correlate with fibrosis. The primary endpoint will be an assessment of fibrosis over the entire liver using multi-parametric magnetic resonance imaging (LiverMultiScan®), which is a validated and proprietary MRI protocol of Perspectum Diagnostics. Published information shows that this method is a sensitive approach to demonstrate differences in the degree of liver fibrosis (1). We chose this as the primary endpoint because data from Perspectum Diagnostics shows there are only small variations in repeated tests in the same individual.
Secondary endpoints will evaluate liver stiffness, which correlates to the degree of liver fibrosis, as assessed by magnetic resonance-elastography (MRE) and FibroScan® (FS). MRE provides a measure of liver stiffness over the entire imaged organ and has high diagnostic accuracy for detection of fibrosis in NASH, independent of BMI and degree of inflammation (2, 3). FS provides a measure of stiffness in a region of liver tissue about 100 times larger than a liver biopsy and also has good diagnostic accuracy for detecting liver fibrosis (4). These three tests will provide information on drug effect, as well as on the performance of the three tests relative to one another, which may indicate the best test(s) to utilize in future studies.
How will the results of the NASH-FX trial advance GR-MD-02 toward approval? With successful improvement in non-invasive fibrosis assessments, we will have proof-of-concept that treatment with GR-MD-02 over four months may be useful in this patient population. Because we are using actual physical liver measurements that correlate with fibrosis, positive study results will significantly improve the odds of success with future clinical trials and inform the dose and duration of treatment moving forward. While this trial alone will not be sufficient for drug approval for this indication, it will be critical for the design of pivotal trials for the purpose of marketing approval. We anticipate results for the NASH-FX trial will be available in the third quarter of 2016.
These “CEO Perspectives” will be a regular feature of our communication activities and may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events and use words such as “may,” “could,” “expect” and others. These statements include those regarding the hope that Galectin’s development program for GR-MD-02 will lead to the first therapy for the treatment of fatty liver disease with advanced fibrosis and cirrhosis. For a discussion of additional factors impacting Galectin’s business, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, and subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause its views to change, management disclaims any obligation to update forward-looking statements
Please look for future editions in which multiple aspects of our development programs for unmet medical needs will be addressed.
Reference List
1. Banerjee R, Pavlides M, Tunnicliffe EM, Piechnik SK, Sarania N, Philips R, et al. Multiparametric magnetic resonance for the non-invasive diagnosis of liver disease. J Hepatol 2014 Jan;60(1):69-77.
2. Singh S, Venkatesh SK, Loomba R, Wang Z, Sirlin C, Chen J, et al. Magnetic resonance elastography for staging liver fibrosis in non-alcoholic fatty liver disease: a diagnostic accuracy systematic review and individual participant data pooled analysis. Eur Radiol 2015 Aug 28.
3. Singh S, Venkatesh SK, Wang Z, Miller FH, Motosugi U, Low RN, et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: a systematic review and meta-analysis of individual participant data. Clin Gastroenterol Hepatol 2015 Mar;13(3):440-451.
4. Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le BB, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010 Feb;51(2):454-462.
Make a Comment or Ask a Question
[contact-form-7]The post Second Phase 2 clinical trial initiated for fatty liver disease with advanced fibrosis appeared first on NASH & Liver Fibrosis | Galectin Therapeutics.
]]>